Journal of Rare Earths ( IF 5.2 ) Pub Date : 2020-11-07 , DOI: 10.1016/j.jre.2020.11.002 Sheng Chang , Mei Li , Kai Gao , Dongliang Zhang , Haijun Duan , Linlin Ma , Zheng Ruan
|
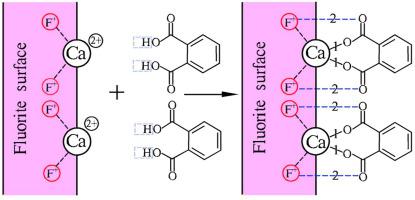
The mechanism of phthalic acid, a dicarboxylic acid collector, in flotation separation of fluorite and rare earth (RE) was studied in this paper. The experimental data of flotation show that phthalic acid, as the collector, can realize highly efficient separation of fluorite and rare earth under weakly acidic conditions. The adsorption mechanism of phthalic acid on the surface of fluorite and bastnaesite was analyzed in this paper by means of the zeta potential measurement, the Fourier transform infrared (FT-IR), the X-ray photoelectron spectroscopy (XPS) and the stability constant measurement of active metal ion and phthalic acid coordination complex. According to the zeta potential testing results, the surfaces of fluorite adsorb the collector phthalate ion with negative charge under weakly acidic conditions which, in turn, increases its electronegativity and results in the motion of its potential. After the reaction between phthalic acid and fluorite ores under weakly acidic conditions, the peak of the fluorite ores is found to have significant changes in the FT-IR results, indicating strong chemical adsorption on the surfaces of phthalic acid and fluorite ores. According to the XPS analysis, the peak of benzene ring of phthalic acid is as high as 2% on the surface of fluorite, while no obvious characteristic peak of benzene ring is found on the surface of bastnaesite. According to the pH potentiometric titration results, the stability constant Ktotal of calcium phthalate complex within the acid range is higher than the stability constant Ktotal' of cerium phthalate complex, indicating that the complex generated between phthalic acid and Ca2+ is more stable than the complex generated between phthalic acid and Ce3+. The possible reason is that Ca2+, with the highest reticular density, plays a prevailing role in the octahedron structure of fluorite amidst the acidic media. As the active point of flotation, Ca2+ works with the carboxyl groups of the collector phthalic acid (–C=O–) to form polycyclic calcium phthalate complex.
中文翻译:

萤石和稀土浮选分离邻苯二甲酸捕收剂的机理
研究了邻苯二甲酸作为二元羧酸捕收剂在萤石和稀土(RE)浮选分离中的作用机理。浮选实验数据表明,邻苯二甲酸作为捕收剂,可以在弱酸性条件下实现萤石和稀土的高效分离。本文通过zeta电位测量、傅里叶变换红外光谱(FT-IR)、X射线光电子能谱(XPS)和稳定性常数测量等手段分析了邻苯二甲酸在萤石和氟碳铈矿表面的吸附机理。活性金属离子与邻苯二甲酸配位络合物。根据zeta电位测试结果,萤石表面在弱酸性条件下吸附带负电荷的邻苯二甲酸盐离子,反过来,增加其电负性并导致其电位运动。邻苯二甲酸与萤石矿在弱酸性条件下反应后,FT-IR结果发现萤石矿的峰有显着变化,表明邻苯二甲酸与萤石矿表面有强烈的化学吸附。根据XPS分析,萤石表面的邻苯二甲酸苯环峰高达2%,而氟碳铈矿表面未发现明显的苯环特征峰。根据 pH 电位滴定结果,稳定常数 FT-IR结果发现萤石矿石的峰有显着变化,表明邻苯二甲酸和萤石矿石表面有强烈的化学吸附。根据XPS分析,萤石表面的邻苯二甲酸苯环峰高达2%,而氟碳铈矿表面未发现明显的苯环特征峰。根据 pH 电位滴定结果,稳定常数 FT-IR结果发现萤石矿石的峰有显着变化,表明邻苯二甲酸和萤石矿石表面有强烈的化学吸附。根据XPS分析,萤石表面的邻苯二甲酸苯环峰高达2%,而氟碳铈矿表面未发现明显的苯环特征峰。根据 pH 电位滴定结果,稳定常数ķ总邻苯二甲酸钙络合物的酸的范围内比所述稳定常数更高ķ总邻苯二甲酸铈的'复合体,这表明复合苯二甲酸之间产生和Ca 2+比邻苯二甲酸和Ce之间产生的复杂的更稳定3+ . 可能的原因是Ca 2+具有最高的网状密度,在酸性介质中萤石的八面体结构中起主导作用。作为浮选的活性点,Ca 2+与捕收剂邻苯二甲酸(–C=O–)的羧基作用形成多环邻苯二甲酸钙络合物。









































 京公网安备 11010802027423号
京公网安备 11010802027423号