Journal of Energy Chemistry ( IF 14.0 ) Pub Date : 2020-11-09 , DOI: 10.1016/j.jechem.2020.11.001 Helen Maria Joseph , Maximilian Fichtner , Anji Reddy Munnangi
|
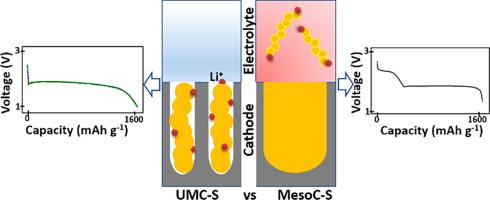
Lithium–sulphur (Li–S) batteries are currently considered as next-generation battery technology. Sulphur is an attractive positive electrode for lithium metal batteries, mainly due to its high capacity (1675 mAh g−1) and high specific energy (2600 Wh kg−1). The electrochemical reaction of lithium with sulphur in non-aqueous electrolytes results in the formation of electrolyte soluble intermediate lithium–polysulphides. The dissolved polysulphides shuttle to the anode and get reduced at the anode resulting in Li metal corrosion. The solubility of polysulphide gradually reduces the amount of sulphur in the cathode, thereby limiting the cycle life of Li–S batteries. Several strategies have been proposed to improve the cycling stability of Li–S batteries. A unique approach to eliminate the polysulphide shuttle is to use ultramicroporous carbon (UMC) as a host for sulphur. The pore size of UMC which is below 7 Å, is the bottleneck for carbonate solvents to access sulphur/polysulphides confined in the pores, thereby preventing the polysulphide dissolution. This perspective article will emphasise the role of UMC host in directing the lithiation mechanism of sulphur and in inhibiting polysulphide dissolution, including the resulting parasitic reaction on the lithium anode. Further, the challenges that need to be addressed by UMC-S based Li–S batteries, and the strategies to realise high power density, high Coulombic efficiency, and resilient Li–S batteries will be discussed.
中文翻译:

超微孔碳作为Li–S电池的硫基质的前景
锂硫(Li–S)电池目前被视为下一代电池技术。硫是锂金属电池有吸引力的正极,主要是由于其高容量(1675 mAh g -1)和高比能(2600 Wh kg -1))。非水电解质中锂与硫的电化学反应导致形成可溶于电解质的中间锂多硫化物。溶解的多硫化物穿梭至阳极并在阳极处还原,导致锂金属腐蚀。多硫化物的溶解度逐渐降低了阴极中硫的含量,从而限制了Li-S电池的循环寿命。已经提出了几种提高Li-S电池循环稳定性的策略。消除多硫化物穿梭的独特方法是使用超微孔碳(UMC)作为硫的主体。低于7的UMC孔径是碳酸盐溶剂进入限制在孔中的硫/多硫化物的瓶颈,从而阻止了多硫化物的溶解。该观点文章将强调UMC主体在指导硫的锂化机理和抑制多硫化物溶解(包括在锂阳极上产生的寄生反应)方面的作用。此外,还将讨论基于UMC-S的Li-S电池需要解决的挑战,以及实现高功率密度,高库仑效率和弹性Li-S电池的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号