当前位置:
X-MOL 学术
›
Int. J. Mass Spectrom.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism and Dynamics of CH2NO2− + CCl4 Halophilic Reaction
International Journal of Mass Spectrometry ( IF 1.6 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.ijms.2020.116470 Sayoni Mitra , Siddharth Sankar Dutta , Nishant Sharma , Upakarasamy Lourderaj
International Journal of Mass Spectrometry ( IF 1.6 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.ijms.2020.116470 Sayoni Mitra , Siddharth Sankar Dutta , Nishant Sharma , Upakarasamy Lourderaj
|
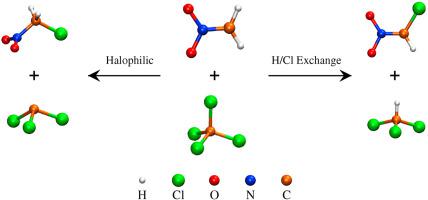
Abstract In halophilic reactions, the nucleophile attacks the halogen moiety of the substrate instead of the carbon atom and displaces a carbanion as the leaving group. We report here a detailed ab initio and DFT study of the mechanism and dynamics of the reaction between CCl4 and C H 2 N O 2 − in the gas phase. The potential energy profiles for the reaction were mapped using DFT, MP2, and CCSD(T) methods considering the bimolecular halophilic (SN2X), SN2, and H/Cl exchange pathways. The SN2X pathway results in the products CCl 3 − + CH2ClNO2, while the H/Cl exchange pathway gives CHCl3 + CHCl N O 2 − . The H/Cl exchange products were also possible by a sequential pathway that involved the SN2X reaction followed by a proton transfer from CH2ClNO2 to CCl 3 − . The SN2X and the sequential H/Cl exchange pathways were found to be barrierless and energetically feasible. The atomic-level mechanisms followed in the reaction was investigated by on-the-fly classical trajectory simulations at the B3LYP/6-311++G** level of theory. The trajectory simulations support the formation of both the halophilic and H/Cl exchange products. The halophilic reaction was found to largely follow non-IRC dynamical pathway that avoids the pre-transition state complex. In addition, the dynamics of the reaction was found to be nonstatistical with the trajectories showing recrossing behavior and nonexponential lifetimes for the post-transition state complex that leads to the halophilic products.
中文翻译:

CH2NO2− + CCl4 嗜盐反应的机理和动力学
摘要 在亲盐反应中,亲核试剂攻击底物的卤素部分而不是碳原子,并取代作为离去基团的碳负离子。我们在此报告了 CCl4 和 CH 2 NO 2 - 在气相中的反应机理和动力学的详细从头算和 DFT 研究。考虑到双分子嗜盐 (SN2X)、SN2 和 H/Cl 交换途径,使用 DFT、MP2 和 CCSD(T) 方法绘制了反应的势能分布图。SN2X 途径产生产物 CCl 3 - + CH2ClNO2,而 H/Cl 交换途径产生 CHCl3 + CHCl NO 2 - 。H/Cl 交换产物也可能通过涉及 SN2X 反应的顺序途径,然后质子从 CH2ClNO2 转移到 CCl 3 - 。SN2X 和连续的 H/Cl 交换途径被发现是无障碍的并且在能量上是可行的。通过 B3LYP/6-311++G** 理论水平的动态经典轨迹模拟研究了反应中遵循的原子级机制。轨迹模拟支持嗜盐和 H/Cl 交换产物的形成。发现嗜盐反应在很大程度上遵循非 IRC 动力学途径,避免了过渡前复合物。此外,发现反应动力学是非统计的,轨迹显示出导致嗜盐产物的过渡态复合物的重新交叉行为和非指数寿命。通过 B3LYP/6-311++G** 理论水平的动态经典轨迹模拟研究了反应中遵循的原子级机制。轨迹模拟支持嗜盐和 H/Cl 交换产物的形成。发现嗜盐反应在很大程度上遵循非 IRC 动力学途径,避免了过渡前复合物。此外,发现反应动力学是非统计的,轨迹显示出导致嗜盐产物的过渡态复合物的重新交叉行为和非指数寿命。通过 B3LYP/6-311++G** 理论水平的动态经典轨迹模拟研究了反应中遵循的原子级机制。轨迹模拟支持嗜盐和 H/Cl 交换产物的形成。发现嗜盐反应在很大程度上遵循非 IRC 动力学途径,避免了过渡前复合物。此外,发现反应动力学是非统计的,轨迹显示出导致嗜盐产物的过渡态复合物的重新交叉行为和非指数寿命。发现嗜盐反应在很大程度上遵循非 IRC 动力学途径,避免了过渡前复合物。此外,发现反应动力学是非统计的,轨迹显示出导致嗜盐产物的过渡态复合物的重新交叉行为和非指数寿命。发现嗜盐反应主要遵循非 IRC 动力学途径,避免了过渡态前复合物。此外,发现反应动力学是非统计的,轨迹显示出导致嗜盐产物的过渡态复合物的重新交叉行为和非指数寿命。
更新日期:2021-01-01
中文翻译:

CH2NO2− + CCl4 嗜盐反应的机理和动力学
摘要 在亲盐反应中,亲核试剂攻击底物的卤素部分而不是碳原子,并取代作为离去基团的碳负离子。我们在此报告了 CCl4 和 CH 2 NO 2 - 在气相中的反应机理和动力学的详细从头算和 DFT 研究。考虑到双分子嗜盐 (SN2X)、SN2 和 H/Cl 交换途径,使用 DFT、MP2 和 CCSD(T) 方法绘制了反应的势能分布图。SN2X 途径产生产物 CCl 3 - + CH2ClNO2,而 H/Cl 交换途径产生 CHCl3 + CHCl NO 2 - 。H/Cl 交换产物也可能通过涉及 SN2X 反应的顺序途径,然后质子从 CH2ClNO2 转移到 CCl 3 - 。SN2X 和连续的 H/Cl 交换途径被发现是无障碍的并且在能量上是可行的。通过 B3LYP/6-311++G** 理论水平的动态经典轨迹模拟研究了反应中遵循的原子级机制。轨迹模拟支持嗜盐和 H/Cl 交换产物的形成。发现嗜盐反应在很大程度上遵循非 IRC 动力学途径,避免了过渡前复合物。此外,发现反应动力学是非统计的,轨迹显示出导致嗜盐产物的过渡态复合物的重新交叉行为和非指数寿命。通过 B3LYP/6-311++G** 理论水平的动态经典轨迹模拟研究了反应中遵循的原子级机制。轨迹模拟支持嗜盐和 H/Cl 交换产物的形成。发现嗜盐反应在很大程度上遵循非 IRC 动力学途径,避免了过渡前复合物。此外,发现反应动力学是非统计的,轨迹显示出导致嗜盐产物的过渡态复合物的重新交叉行为和非指数寿命。通过 B3LYP/6-311++G** 理论水平的动态经典轨迹模拟研究了反应中遵循的原子级机制。轨迹模拟支持嗜盐和 H/Cl 交换产物的形成。发现嗜盐反应在很大程度上遵循非 IRC 动力学途径,避免了过渡前复合物。此外,发现反应动力学是非统计的,轨迹显示出导致嗜盐产物的过渡态复合物的重新交叉行为和非指数寿命。发现嗜盐反应在很大程度上遵循非 IRC 动力学途径,避免了过渡前复合物。此外,发现反应动力学是非统计的,轨迹显示出导致嗜盐产物的过渡态复合物的重新交叉行为和非指数寿命。发现嗜盐反应主要遵循非 IRC 动力学途径,避免了过渡态前复合物。此外,发现反应动力学是非统计的,轨迹显示出导致嗜盐产物的过渡态复合物的重新交叉行为和非指数寿命。











































 京公网安备 11010802027423号
京公网安备 11010802027423号