European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-11-07 , DOI: 10.1016/j.ejmech.2020.112999 Yancheng Yu , Quanwei Yu , Simeng Liu , Chenyang Wu , Xiaojin Zhang
|
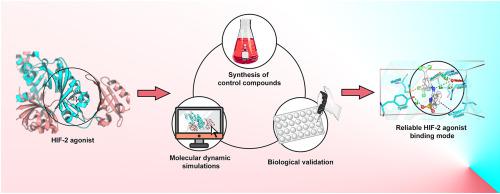
Hypoxia-inducible factor-2 (HIF-2), a heterodimeric transcriptional protein consisting of HIF-2α and aryl hydrocarbon receptor nuclear translocator (ARNT) subunits, has a broad transcriptional profile that plays a vital role in human oxygen metabolism. M1001, a HIF-2 agonist identified by high-throughput screening (HTS), is capable of altering the conformation of Tyr281 of the HIF-2α PAS-B domain and enhancing the affinity of HIF-2α and ARNT for transcriptional activation. M1002, an analog of M1001, shows improved efficacy than M1001. However, the cocrystal structure of M1001 and HIF-2 has some defects in revealing the agonist binding mode due to the relatively low resolution, while the binding mode of M1002 remained unexplored. To in-depth understand agonist binding profiles, herein, the molecular dynamic (MD) simulations was applied to construct a stable agonist-protein model, and a possible binding mode was proposed through the analysis of the binding free energy and hydrogen bonding of the simulation results. Nine compounds were then synthesized and evaluated to verify the proposed binding mode. Among them, compound 10 manifested improved agonistic activity and reduced toxicity compared to M1002. This study provides deep insight into the binding mode of such HIF-2 agonists, which would be useful for designing novel agonists for HIF-2.
中文翻译:

通过分子动力学模拟和生物学验证了解HIF-2激动剂的结合模式
缺氧诱导因子2(HIF-2)是由HIF-2α和芳烃受体核转运子(ARNT)亚基组成的异二聚体转录蛋白,具有广泛的转录谱,在人类氧代谢中起着至关重要的作用。通过高通量筛选(HTS)鉴定的HIF-2激动剂M1001能够改变HIF-2αPAS-B结构域的Tyr281构象,并增强HIF-2α和ARNT对转录激活的亲和力。M1002是M1001的类似物,显示出比M1001更高的功效。然而,由于相对较低的分辨率,M1001和HIF-2的共晶体结构在揭示激动剂结合模式方面存在一些缺陷,而M1001和HIF-2的结合模式则存在一些缺陷。M1002尚未开发。为了深入了解激动剂的结合特性,本文采用分子动力学(MD)模拟方法建立了稳定的激动剂-蛋白质模型,并通过分析结合能和氢键,提出了可能的结合方式。结果。然后合成了九种化合物并进行了评估,以验证提出的结合模式。其中,与M1002相比,化合物10表现出改善的激动活性和降低的毒性。这项研究提供了深入了解这种HIF-2激动剂的结合模式,这对于设计新型的HIF-2激动剂很有用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号