Clinical Immunology ( IF 4.5 ) Pub Date : 2020-11-08 , DOI: 10.1016/j.clim.2020.108620 Kalliopi Domvri , Savvas Petanidis , Paul Zarogoulidis , Doxakis Anestakis , Drosos Tsavlis , Chong Bai , Haidong Huang , Lutz Freitag , Wolfgang Hohenforst-Schmidt , Konstantinos Porpodis , Theodora Katopodi
|
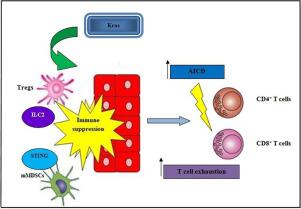
Lung cancer remains the leading cause of cancer-related deaths and despite extensive research, the survival rate of lung cancer patients remains significantly low. Recent data reveal that aberrant Kras signaling drives regulatory T cells (Tregs) present in lung tumor microenvironment to establish immune deregulation, and immunosuppression but the exact pathogenic mechanism is still unknown. In this study, we investigate the role of oncogenic Kras in Treg-related immunosuppression and its involvement in tumor-associated metabolic reprogramming. Findings reveal Tregs to prompt GATA3/NOS2-related immunosuppression via STING inhibition which triggers a decline in CD4+ T infiltration, and a subsequent increase in lung metastatic burden. Enhanced Treg expression was also associated with low T/MDSC ratio through restriction of CD8+CD44+CD62L− T effector cells, contributing to a tumor-promoting status. Specifically, TIM3+/LAG3+ Tregs prompted Kras-related immunosuppressive chemoresistance and were associated with T cell dysfunction. This Treg-dependent immunosuppression correlated with CD8 T cell exhaustion phenotype and ILC2 augmentation in mice. Moreover, enhanced Treg expression promoted activation-induced cell death (AICD) of T lymphocytes and guided lymph node metastasis in vivo. Overall, these findings demonstrate the multifaceted roles of Tregs in sustaining lung immunosuppressive neoplasia through tumor microenvironment remodeling and provide new opportunities for effective metastasis inhibition, especially in chemoresistant tumors.
中文翻译:

Treg依赖性免疫抑制通过STING / ILC2轴触发效应子T细胞功能障碍
肺癌仍然是癌症相关死亡的主要原因,尽管进行了广泛的研究,肺癌患者的存活率仍然很低。最近的数据显示,异常的Kras信号传导驱动肺肿瘤微环境中存在的调节性T细胞(Tregs)建立免疫失调和免疫抑制作用,但确切的致病机制仍不清楚。在这项研究中,我们调查致癌性Kras在Treg相关免疫抑制中的作用及其在肿瘤相关代谢重编程中的作用。研究发现,Treg通过抑制STING来促进GATA3 / NOS2相关的免疫抑制,从而触发CD4 +的下降T浸润,随后增加了肺转移负担。通过限制CD8 + CD44 + CD62L - T效应细胞,增强的Treg表达还与低T / MDSC比相关,从而促进了肿瘤的生长。具体来说,TIM3 + / LAG3 +Tregs引起Kras相关的免疫抑制化学耐药,并与T细胞功能障碍有关。这种Treg依赖性免疫抑制与小鼠CD8 T细胞衰竭表型和ILC2增强有关。此外,增强的Treg表达促进体内T淋巴细胞激活诱导的细胞死亡(AICD)和引导的淋巴结转移。总体而言,这些发现表明,Tregs通过肿瘤微环境重塑在维持肺免疫抑制性肿瘤形成中具有多方面的作用,并提供了有效抑制转移的新机会,尤其是在化学耐药性肿瘤中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号