Chemical Engineering Research and Design ( IF 3.7 ) Pub Date : 2020-11-09 , DOI: 10.1016/j.cherd.2020.10.035 Michał Kotowicz , Magdalena Jasińska
|
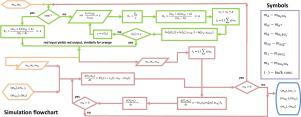
The Villermaux–Dushman test reaction for micromixing characterization has been used by many researchers to investigate homogeneous mixing systems. Due to the complexity of the iodine-forming reaction, the strong effect of ionic strength of reaction medium on its reaction rate constant, as well as the dependence of the sulfuric acid dissociation on its concentration, the kinetic model of the iodide/iodate test reaction with sulfuric acid is complicated. Therefore, a quantitative evaluation of such a test reaction is challenging and remains an open problem. In this paper, the pH of the sulfuric acid solutions with different values of ionic strength is investigated using the model of Pitzer. The numerical results of the simulation are compared with the experimental data and good agreement is obtained. It is also shown that simple equilibrium calculations for sulfuric acid do not give correct pH results when the ionic strength of its solution is increased by the addition of sulfate salts. However, such an assumption yields satisfactory results for the cases without additional salt and with an acid concentration lower than 0.2 M. Finally, the engulfment model of micromixing using activities to calculate proton concentrations is applied to analyze the experimental results from previous papers employing the iodide/iodate method.
中文翻译:

碘化物-碘酸盐法和硫酸微混合实验解释的改进模型
用于微混合特性的Villermaux-Dushman测试反应已被许多研究人员用来研究均质混合系统。由于碘形成反应的复杂性,反应介质的离子强度对其反应速率常数的强烈影响,以及硫酸离解对其浓度的依赖性,碘化物/碘酸盐测试反应的动力学模型用硫酸是复杂的。因此,对这种测试反应进行定量评估具有挑战性,并且仍然是一个未解决的问题。本文使用Pitzer模型研究了具有不同离子强度值的硫酸溶液的pH。将数值模拟结果与实验数据进行比较,取得了很好的一致性。还表明,当硫酸盐溶液的离子强度通过添加硫酸盐而增加时,硫酸的简单平衡计算无法得出正确的pH结果。但是,对于没有添加盐和酸浓度低于0.2 M的情况,这种假设会产生令人满意的结果。最后,使用活性计算质子浓度的微混合的吞吐模型用于分析以前使用碘化物的论文的实验结果/ iodate方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号