Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2020-11-09 , DOI: 10.1016/j.cej.2020.127656 E. Benhelal , J.M. Hook , M.I. Rashid , G. Zhao , T.K. Oliver , M.S. Rayson , G.F. Brent , M. Stockenhuber , E.M. Kennedy
|
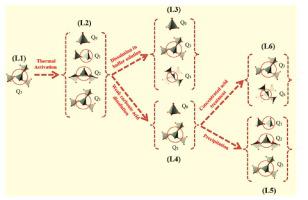
This work investigates chemical stability of Mg-silicates and silica in aqueous systems in order to gain in-depth understanding of their dissolution and precipitation behaviour under different conditions. The aim was to utilise the knowledge gained to develop an engineered carbon mineralisation technology for an efficient and cost competitive CO2 capture and utilisation. The results, based firmly on 29Si solid-state MAS NMR spectroscopy, and complementary techniques, demonstrate the influence of Si coordination (Qn) on the extent of Mg-silicates dissolution, as the increase in the number of neighbouring Si atoms in the structure of Mg-silicates reduced dissolution of Mg-silicates. While 100 and 80% of Q1(3Mg) Mg-silicate dissolved in pH=5 and 6.5, the extent of dissolution for Q2(2Mg) was lower at values of 90 and 65% under the same conditions, while Q3(1Mg) did not actually dissolve in solutions with pH=5 and 6.5. The results of precipitation studies indicated the effect of Mg solubility on the structure and Mg content of the precipitated phases. While Mg-silicates with all three structures of Q1(3Mg), Q2(2Mg) and Q3(1Mg) precipitated in the mildly alkaline environment (pH=8.5) with the ratios of 20, 40 and 40% respectively, in concentrated acidic solutions of pH=0, only pure silica (no Mg content) with Q3(1H)/Q4(0H, 0Mg) having a ratio of 35 and 65% precipitated. The results of direct and indirect carbon mineralisation experiments showed that only 40 and 57 wt% of Mg content of thermally treated Mg-silicate was extracted respectively, consistent with 29Si NMR analyses, indicating that only intermediate Mg-silicate phases I and II with Q1(3Mg) and Q2(2Mg) structures were reactive, while other Mg-silicate phases remained inert. Another reason for limited Mg extraction in direct and indirect carbon mineralisation experiments is related to precipitation of a silica-rich phase/s on the surface of the reacting particles leading to passivation, again consistent with 29Si NMR analyses. This confirmed precipitation of Mg-silicate with a Q3(1Mg) structure as well as hydrated silica Q3(1H) and silica Q4(0H, 0Mg) in aqueous environments, similar to carbonation processes.
中文翻译:

使用25 Mg和29 Si固态MAS NMR光谱分析对水体系中镁硅酸盐和二氧化硅的化学稳定性的了解 :CO 2捕集和利用的应用
这项工作研究了Mg-硅酸盐和二氧化硅在水体系中的化学稳定性,以便深入了解它们在不同条件下的溶解和沉淀行为。目的是利用所获得的知识来开发工程碳矿化技术,以实现高效且具有成本竞争力的CO 2捕集和利用。牢固地基于29 Si固态MAS NMR光谱和互补技术的结果表明,随着结构中相邻Si原子数量的增加,Si配位(Qn)对Mg-硅酸盐溶解程度的影响。镁硅酸盐减少了镁硅酸盐的溶解。而Q 1的100%和80%(3Mg)溶解在pH = 5和6.5的Mg-硅酸盐,在相同条件下Q 2(2Mg)的溶解程度在90和65%时较低,而Q 3(1Mg)实际上不溶解在溶液中pH = 5和6.5。沉淀研究的结果表明,Mg溶解度对沉淀相的结构和Mg含量有影响。当具有Q 1(3Mg),Q 2(2Mg)和Q 3(1Mg)三种结构的Mg硅酸盐在温和碱性环境(pH = 8.5)中分别以20%,40%和40%的比例沉淀时。 pH = 0的浓酸性溶液,仅Q 3(1H)/ Q 4的纯二氧化硅(不含Mg)比率为35和65%的(0H,0Mg)析出。直接和间接碳矿化实验的结果表明,热处理的Mg-硅酸盐分别仅提取40%和57 wt%的Mg含量,与29 Si NMR分析一致,表明只有中间的Mg-硅酸盐相I和II与Q 1(3Mg)和Q 2(2Mg)结构呈反应性,而其他Mg-硅酸盐相保持惰性。在直接和间接碳矿化实验中限制Mg萃取的另一个原因与反应颗粒表面上富二氧化硅相的沉淀导致钝化有关,这再次与29 Si NMR分析一致。这证实了具有Q的硅酸镁沉淀类似于碳酸化过程,在水性环境中,其结构为3(1Mg)以及水合二氧化硅Q 3(1H)和二氧化硅Q 4(0H,0Mg)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号