Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2020-11-09 , DOI: 10.1016/j.cej.2020.127658 Defan Yao , Yanshu Wang , Rongfeng Zou , Kexin Bian , Shuguang Yuan , Bingbo Zhang , Dengbin Wang
|
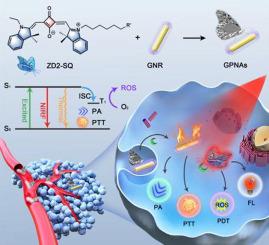
Triple-negative breast cancer (TNBC), as the most tough subtype in breast cancer, has the worst outcome, and developing effective and safe strategies for patients undergone TNBC are urgently needed. Recently, phototheranostics, including optical imaging and phototherapy have shown a great potential in TNBC management. However, most of the phototheranostic probes, as ‘always-on’ agents, lead to high imaging background and nonspecific phototoxicity in adjacent healthy tissues. Herein, a toolbox of wavelength-adjustable butterfly molecules and gold nanorods (GNRs)-based peptide nanoassemblies (GPNAs) were achieved through simple synthesis and stepwise self-assembly for the targeted optical imaging and phototherapeutic of TNBC. Specifically, the self-assembled GPNAs were not only nonfluorescent but also photodynamic-inactivated, which significantly reduces background fluorescence and phototoxicity in adjacent healthy tissue. Extradomain-B fibronectin, as a biomarker of TNBC, can selectively disassemble peptide-conjugated butterfly molecules (ZD2-SQ) from GPNAs and liberate extremely bright NIR fluorescence in high signal-to-background ratio (SBR) and specific photodynamic therapy (PDT) capability while GNRs hold robust photoacoustic (PA) and CT imaging signals as internal reference and convert NIR light into heat energy for photothermal therapy (PTT) of TNBC. This research offers a novel general strategy for the development of activated molecular probes for specific phototheranostics of TNBC.
中文翻译:

波长可调节蝴蝶分子的动态纳米组件中的域外B纤连蛋白调节光学成像和三阴性乳腺癌同步光疗。
三阴性乳腺癌(TNBC)作为乳腺癌中最难治的亚型,其结果最差,因此迫切需要为TNBC患者制定有效且安全的策略。最近,包括光学成像和光疗在内的光热疗法在TNBC管理中显示出巨大的潜力。但是,大多数光热敏探针作为“始终在线”的试剂,会导致高成像背景和相邻健康组织中的非特异性光毒性。在这里,通过简单的合成和逐步自组装的TNBC的目标光学成像和光疗,实现了波长可调的蝴蝶分子和金纳米棒(GNRs)基肽纳米组件(GPNAs)的工具箱。具体来说,自组装的GPNA不仅是非荧光的,而且是光动力灭活的,可以显着降低邻近健康组织的背景荧光和光毒性。域外B纤连蛋白作为TNBC的生物标志物,可以选择性地从GPNA上解离肽缀合的蝴蝶分子(ZD2-SQ),并以高信噪比(SBR)和特异性光动力疗法(PDT)释放极亮的NIR荧光。 GNR具有强大的光声(PA)和CT成像信号作为内部参考,并将NIR光转换为热能以用于TNBC的光热疗法(PTT)。这项研究为开发用于TNBC特定光热学的活化分子探针提供了一种新颖的一般策略。可以选择性地从GPNA上拆解肽偶联的蝴蝶分子(ZD2-SQ),并以高的信噪比(SBR)和特定的光动力疗法(PDT)释放极亮的NIR荧光,而GNR具有强大的光声(PA)和CT成像信号作为内部参考,并将NIR光转换为热能,用于TNBC的光热疗法(PTT)。这项研究为开发用于TNBC特定光热学的活化分子探针提供了一种新颖的一般策略。可以选择性地从GPNA上拆解肽偶联的蝴蝶分子(ZD2-SQ),并以高的信噪比(SBR)和特定的光动力疗法(PDT)释放极亮的NIR荧光,而GNR具有强大的光声(PA)和CT成像信号作为内部参考,并将NIR光转换为热能,用于TNBC的光热疗法(PTT)。这项研究为开发用于TNBC特定光热学的活化分子探针提供了一种新颖的一般策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号