Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2020-11-07 , DOI: 10.1016/j.bmc.2020.115859 Johan Wannberg 1 , Johan Gising 2 , Jens Lindman 2 , Jessica Salander 3 , Hugo Gutiérrez-de-Terán 3 , Hanin Ablahad 4 , Selin Hamid 4 , Alfhild Grönbladh 5 , Iresha Spizzo 6 , Tracey A Gaspari 6 , Robert E Widdop 6 , Anders Hallberg 7 , Maria Backlund 8 , Anna Leśniak 9 , Mathias Hallberg 5 , Mats Larhed 10
|
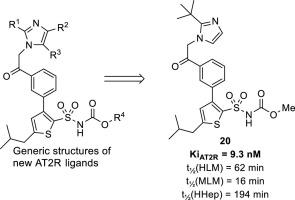
A series of meta-substituted acetophenone derivatives, encompassing N-(alkyloxycarbonyl)thiophene sulfonamide fragments have been synthesized. Several selective AT2 receptor ligands were identified, among those a tert-butylimidazole derivative (20) with a Ki of 9.3 nM, that demonstrates a high stability in human liver microsomes (t½ = 62 min) and in human hepatocytes (t½= 194 min). This methyloxycarbonylthiophene sulfonamide is a 20-fold more potent binder to the AT2 receptor and is considerably more stable in human liver microsomes, than a previously reported and broadly studied structurally related AT2R prototype antagonist 3 (C38). Ligand 20 acts as an AT2R agonist and caused an AT2R mediated concentration-dependent vasorelaxation of pre-contracted mouse aorta. Furthermore, in contrast to imidazole derivative C38, the tert-butylimidazole derivative 20 is a poor inhibitor of CYP3A4, CYP2D6 and CYP2C9. It is demonstrated herein that smaller alkyloxycarbonyl groups make the ligands in this series of AT2 selective compounds less prone to degradation and that a high AT2 receptor affinity can be retained after truncation of the alkyloxycarbonyl group. Binding modes of the most potent AT2R ligands were explored by docking calculations combined with molecular dynamics simulations.
中文翻译:

N-(甲氧羰基)噻吩磺酰胺作为高亲和力 AT2 受体配体
已经合成了一系列间位取代的苯乙酮衍生物,包括N -(烷氧基羰基)噻吩磺酰胺片段。鉴定了几种选择性 AT2 受体配体,其中K i为 9.3 nM的叔丁基咪唑衍生物 ( 20 )在人肝微粒体 (t ½ = 62 分钟) 和人肝细胞 (t ½ = 194 分钟)。这种甲氧羰基噻吩磺酰胺是 AT2 受体结合剂的 20 倍,并且在人肝微粒体中的稳定性比先前报道和广泛研究的结构相关的 AT 2 R 原型拮抗剂3强得多(C38)。配体20作为 AT2R 激动剂并引起 AT2R 介导的预收缩小鼠主动脉的浓度依赖性血管舒张。此外,与咪唑衍生物 C38 相比,叔丁基咪唑衍生物20是 CYP3A4、CYP2D6 和 CYP2C9 的弱抑制剂。本文证明,较小的烷氧基羰基使这一系列AT2选择性化合物中的配体更不易降解,并且在烷氧基羰基截断后可以保持高AT2受体亲和力。通过对接计算结合分子动力学模拟探索了最有效的 AT 2 R 配体的结合模式。



























 京公网安备 11010802027423号
京公网安备 11010802027423号