Biochimica et Biophysica Acta (BBA) - General Subjects ( IF 2.8 ) Pub Date : 2020-11-07 , DOI: 10.1016/j.bbagen.2020.129783 Karl Syson , Sibyl F.D. Batey , Steffen Schindler , Rainer Kalscheuer , Stephen Bornemann
|
Background
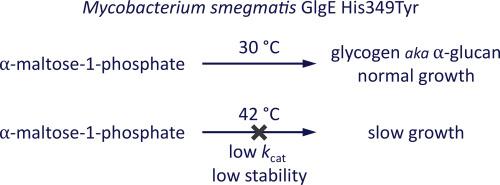
The bacterial GlgE pathway is the third known route to glycogen and is the only one present in mycobacteria. It contributes to the virulence of Mycobacterium tuberculosis. The involvement of GlgE in glycogen biosynthesis was discovered twenty years ago when the phenotype of a temperature-sensitive Mycobacterium smegmatis mutation was rescued by the glgE gene. The evidence at the time suggested glgE coded for a glucanase responsible for the hydrolysis of glycogen, in stark contrast with recent evidence showing GlgE to be a polymerase responsible for its biosynthesis.
Methods
We reconstructed and examined the temperature-sensitive mutant and characterised the mutated GlgE enzyme.
Results
The mutant strain accumulated the substrate for GlgE, α-maltose-1-phosphate, at the non-permissive temperature. The glycogen assay used in the original study was shown to give a false positive result with α-maltose-1-phosphate. The accumulation of α-maltose-1-phosphate was due to the lowering of the kcat of GlgE as well as a loss of stability 42 °C. The reported rescue of the phenotype by GarA could potentially involve an interaction with GlgE, but none was detected.
Conclusions
We have been able to reconcile apparently contradictory observations and shed light on the basis for the phenotype of the temperature-sensitive mutation.
General significance
This study highlights how the lowering of flux through the GlgE pathway can slow the growth mycobacteria.
中文翻译:

温度敏感的耻垢分枝杆菌glgE突变会导致GlgE酶活性和热稳定性的损失以及α-麦芽糖-1-磷酸的积累
背景
细菌GlgE途径是第三个已知的糖原途径,也是分枝杆菌中唯一的途径。它有助于结核分枝杆菌的致病性。20年前,当通过glgE基因挽救了对温度敏感的耻垢分枝杆菌突变的表型时,发现了GlgE参与糖原生物合成。当时的证据表明glgE编码负责糖原水解的葡聚糖酶,与最近的证据表明GlgE是负责其生物合成的聚合酶形成鲜明对比。
方法
我们重建并检查了对温度敏感的突变体,并对突变的GlgE酶进行了表征。
结果
突变菌株在非允许温度下积累了GlgE的底物α-麦芽糖-1-磷酸。原始研究中使用的糖原测定法显示使用α-麦芽糖-1-磷酸产生假阳性结果。α-麦芽糖-1-磷酸的积累是由于GlgE的k cat降低以及42°C的稳定性损失所致。据报道,GarA对表型的拯救可能涉及与GlgE的相互作用,但未检测到。
结论
我们已经能够调和明显矛盾的观察结果,并根据对温度敏感的突变的表型来阐明。
一般意义
这项研究强调了通过GlgE途径的通量降低如何减慢分枝杆菌的生长。











































 京公网安备 11010802027423号
京公网安备 11010802027423号