当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of substituted anilines via a gold-catalyzed three-component reaction
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-11-02 , DOI: 10.1039/d0ob02018d Hirofumi Ueda 1, 2, 3, 4 , Ryota Yamamoto 1, 2, 3, 4 , Minami Yamaguchi 1, 2, 3, 4 , Hidetoshi Tokuyama 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-11-02 , DOI: 10.1039/d0ob02018d Hirofumi Ueda 1, 2, 3, 4 , Ryota Yamamoto 1, 2, 3, 4 , Minami Yamaguchi 1, 2, 3, 4 , Hidetoshi Tokuyama 1, 2, 3, 4
Affiliation
|
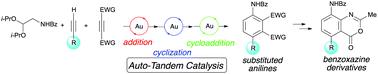
A three-component reaction for the synthesis of substituted anilines by a gold(I)-catalyzed domino reaction was developed. Cationic gold catalysts selectively and sequentially activated two different alkynes, which were involved in pyrrole synthesis and subsequent Diels–Alder reaction. The sequential formal (3 + 2) annulation/Diels–Alder reaction of three components provided a variety of substituted anilines in a modular fashion. Moreover, utility of the aniline products was demonstrated by derivatization to substituted benzoxazines, which are pharmaceutically important heterocycles.
中文翻译:

通过金催化的三组分反应合成取代的苯胺
开发了一种三组分反应,用于通过金(I)催化的多米诺骨牌反应合成取代的苯胺。阳离子金催化剂选择性和顺序地活化了两个不同的炔烃,它们参与了吡咯的合成和随后的Diels-Alder反应。三种组分的顺序形式(3 + 2)正环化/ Diels-Alder反应以模块化的方式提供了多种取代苯胺。此外,苯胺产品的实用性通过衍生为取代的苯并恶嗪而得到证明,后者是药学上重要的杂环。
更新日期:2020-11-06
中文翻译:

通过金催化的三组分反应合成取代的苯胺
开发了一种三组分反应,用于通过金(I)催化的多米诺骨牌反应合成取代的苯胺。阳离子金催化剂选择性和顺序地活化了两个不同的炔烃,它们参与了吡咯的合成和随后的Diels-Alder反应。三种组分的顺序形式(3 + 2)正环化/ Diels-Alder反应以模块化的方式提供了多种取代苯胺。此外,苯胺产品的实用性通过衍生为取代的苯并恶嗪而得到证明,后者是药学上重要的杂环。



























 京公网安备 11010802027423号
京公网安备 11010802027423号