当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Separation of precious metals by split-anion extraction using water-saturated ionic liquids
Green Chemistry ( IF 9.3 ) Pub Date : 2020-11-06 , DOI: 10.1039/d0gc02356f Viet Tu Nguyen 1, 2, 3, 4 , Sofía Riaño 1, 2, 3, 4 , Koen Binnemans 1, 2, 3, 4
Green Chemistry ( IF 9.3 ) Pub Date : 2020-11-06 , DOI: 10.1039/d0gc02356f Viet Tu Nguyen 1, 2, 3, 4 , Sofía Riaño 1, 2, 3, 4 , Koen Binnemans 1, 2, 3, 4
Affiliation
|
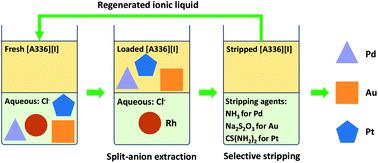
A split-anion solvent extraction process was developed for the separation of precious metal ions Au(III), Pt(IV), Pd(II) and Rh(III) from aqueous chloride media using water-saturated ionic liquids. The metal extraction and stripping behavior of the chloride form [A336][Cl], bromide form [A336][Br] and the iodide form [A336][I] of the quaternary ammonium ionic liquid Aliquat 336 were compared. The three ionic liquids extracted Au(III), Pd(II) and Pt(IV) quantitatively in most cases, whereas the co-extraction of Rh(III) was strongly dependent on the acidity and the chloride concentration. Among the studied ionic liquids, [A336][I] achieved the highest separation factors between Pd(II)/Rh(III), Pt(IV)/Rh(III), and Au(III)/Rh(III) at 6 mol L−1 Cl−. Additionally, the selective stripping of the individual metal ions Pd(II), Au(III), and Pt(IV) was only possible from loaded [A336][I] using ammonia solution (NH4OH), sodium thiosulfate (Na2S2O3), and thiourea ((NH2)2CS), respectively. A closed-loop flow sheet was designed for the recovery of the precious metals from chloride media using split-anion extraction with [A336][I]. The integrated process was demonstrated to be suitable for the purification of Rh(III), Pt(IV) and Pd(II) from a complex metal feed such as the leachate of spent automotive catalysts. The ionic liquid-based split-anion extraction process is simple, selective and effective for the sustainable separation of the precious metals, using only one green extractant [A336][I], which can be regenerated for consecutive extraction-stripping cycles.
中文翻译:

使用水饱和离子液体的分离阴离子萃取分离贵金属
开发了一种分离阴离子溶剂萃取工艺,该工艺使用水饱和离子液体从氯化物水溶液中分离贵金属离子Au(III),Pt(IV),Pd(II)和Rh(III)。比较了季铵离子液体Aliquat 336的氯化物形式[A336] [Cl],溴化物形式[A336] [Br]和碘化物形式[A336] [I]的金属萃取和汽提行为。在大多数情况下,这三种离子液体可定量萃取Au(III),Pd(II)和Pt(IV),而Rh(III)的共萃取)在很大程度上取决于酸度和氯化物浓度。在研究的离子液体中,[A336] [I]在6时达到了Pd(II)/ Rh(III),Pt(IV)/ Rh(III)和Au(III)/ Rh(III)之间最高的分离系数摩尔大号-1氯- 。此外,只有使用氨溶液(NH 4 OH),硫代硫酸钠(Na 2)从负载的[A336] [I]中选择性剥离单个金属离子Pd(II),Au(III)和Pt(IV)S 2 O 3)和硫脲((NH2) 2 CS)。设计了一种闭环流程图,用于使用[A336] [I]的分离阴离子萃取法从氯化物介质中回收贵金属。事实证明,该集成方法适用于从复杂的金属进料(例如废旧汽车催化剂的浸出液)中纯化Rh( III),Pt( IV)和Pd( II)。基于离子液体的分裂阴离子萃取工艺简单,选择性且有效地可持续分离贵金属,仅使用一种绿色萃取剂[A336] [I],即可将其再生以进行连续的萃取-汽提循环。
更新日期:2020-11-06
中文翻译:

使用水饱和离子液体的分离阴离子萃取分离贵金属
开发了一种分离阴离子溶剂萃取工艺,该工艺使用水饱和离子液体从氯化物水溶液中分离贵金属离子Au(III),Pt(IV),Pd(II)和Rh(III)。比较了季铵离子液体Aliquat 336的氯化物形式[A336] [Cl],溴化物形式[A336] [Br]和碘化物形式[A336] [I]的金属萃取和汽提行为。在大多数情况下,这三种离子液体可定量萃取Au(III),Pd(II)和Pt(IV),而Rh(III)的共萃取)在很大程度上取决于酸度和氯化物浓度。在研究的离子液体中,[A336] [I]在6时达到了Pd(II)/ Rh(III),Pt(IV)/ Rh(III)和Au(III)/ Rh(III)之间最高的分离系数摩尔大号-1氯- 。此外,只有使用氨溶液(NH 4 OH),硫代硫酸钠(Na 2)从负载的[A336] [I]中选择性剥离单个金属离子Pd(II),Au(III)和Pt(IV)S 2 O 3)和硫脲((NH2) 2 CS)。设计了一种闭环流程图,用于使用[A336] [I]的分离阴离子萃取法从氯化物介质中回收贵金属。事实证明,该集成方法适用于从复杂的金属进料(例如废旧汽车催化剂的浸出液)中纯化Rh( III),Pt( IV)和Pd( II)。基于离子液体的分裂阴离子萃取工艺简单,选择性且有效地可持续分离贵金属,仅使用一种绿色萃取剂[A336] [I],即可将其再生以进行连续的萃取-汽提循环。











































 京公网安备 11010802027423号
京公网安备 11010802027423号