当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Examination of the Role of Mg2+ in the Mechanism of Nucleotide Binding to the Monomeric YME1L AAA+ Domain
Biochemistry ( IF 2.9 ) Pub Date : 2020-11-06 , DOI: 10.1021/acs.biochem.0c00699 Justin M Miller 1 , Chad A Brambley 1 , Justin D Marsee 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-11-06 , DOI: 10.1021/acs.biochem.0c00699 Justin M Miller 1 , Chad A Brambley 1 , Justin D Marsee 1
Affiliation
|
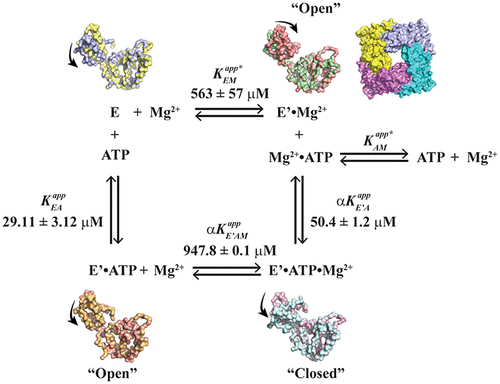
The first line of defense in the mitochondrial quality control network involves the stress response from a family of ATP-dependent proteases. We have reported that a solubilized version of the mitochondrial inner membrane ATP-dependent protease YME1L displays nucleotide binding kinetics that are sensitive to the reactive oxygen species hydrogen peroxide under a limiting ATP concentration. Our observations were consistent with an altered YME1L conformational ensemble leading to increased nucleotide binding site accessibility under oxidative stress conditions. To examine this hypothesis further, we report here the results of a comprehensive study of the thermodynamic and kinetic properties underlying the binding of nucleoside di- and triphosphate to the isolated YME1L AAA+ domain (YME1L-AAA+). A combination of fluorescence titrations, molecular dynamics, and stopped-flow fluorescence experiments have demonstrated similarity between nucleotide binding behaviors for YME1L under oxidative conditions and the isolated AAA+ domain. Our data demonstrate that YME1L-AAA+ binds ATP and ADP with affinities equal to ∼30 and 5 μM, respectively, in the absence of Mg2+. We note a negative heterotropic linkage effect between Mg2+ and ATP that arises as the MgCl2 concentration is increased such that the affinity of YME1L-AAA+ for ATP decreases to ∼60 μM in the presence of 10 mM MgCl2. Molecular dynamics methods allow for structural rationalization by revealing condition-dependent conformational populations for YME1L-AAA+. Taken together, these data suggest a preliminary model in which YME1L modulates its affinity for the nucleotide to stabilize against degradation or instability inherent to such stress conditions.
中文翻译:

检查 Mg2+ 在核苷酸与单体 YME1L AAA+ 结构域结合的机制中的作用
线粒体质量控制网络中的第一道防线涉及 ATP 依赖性蛋白酶家族的应激反应。我们报道了线粒体内膜 ATP 依赖性蛋白酶 YME1L 的溶解版本显示出在限制 ATP 浓度下对活性氧过氧化氢敏感的核苷酸结合动力学。我们的观察结果与 YME1L 构象整体的改变一致,导致氧化应激条件下核苷酸结合位点的可及性增加。为了进一步检验这一假设,我们在此报告了对核苷二磷酸和三磷酸与分离的 YME1L AAA+ 结构域 (YME1L-AAA+) 结合的热力学和动力学特性的综合研究结果。荧光滴定、分子动力学和停流荧光实验的结合证明了氧化条件下 YME1L 的核苷酸结合行为与分离的 AAA+ 结构域之间的相似性。我们的数据表明,在没有 Mg 2+的情况下,YME1L-AAA+ 结合 ATP 和 ADP 的亲和力分别为 ∼30 和 5 μM。我们注意到,随着 MgCl 2浓度的增加,Mg 2+和 ATP 之间出现负异向连接效应,从而在 10 mM MgCl 2存在的情况下,YME1L-AAA+ 对 ATP 的亲和力降低至~60 μM。分子动力学方法通过揭示 YME1L-AAA+ 的条件依赖性构象群来实现结构合理化。总而言之,这些数据表明了一个初步模型,其中 YME1L 调节其对核苷酸的亲和力,以稳定对抗此类应激条件固有的降解或不稳定性。
更新日期:2020-11-17
中文翻译:

检查 Mg2+ 在核苷酸与单体 YME1L AAA+ 结构域结合的机制中的作用
线粒体质量控制网络中的第一道防线涉及 ATP 依赖性蛋白酶家族的应激反应。我们报道了线粒体内膜 ATP 依赖性蛋白酶 YME1L 的溶解版本显示出在限制 ATP 浓度下对活性氧过氧化氢敏感的核苷酸结合动力学。我们的观察结果与 YME1L 构象整体的改变一致,导致氧化应激条件下核苷酸结合位点的可及性增加。为了进一步检验这一假设,我们在此报告了对核苷二磷酸和三磷酸与分离的 YME1L AAA+ 结构域 (YME1L-AAA+) 结合的热力学和动力学特性的综合研究结果。荧光滴定、分子动力学和停流荧光实验的结合证明了氧化条件下 YME1L 的核苷酸结合行为与分离的 AAA+ 结构域之间的相似性。我们的数据表明,在没有 Mg 2+的情况下,YME1L-AAA+ 结合 ATP 和 ADP 的亲和力分别为 ∼30 和 5 μM。我们注意到,随着 MgCl 2浓度的增加,Mg 2+和 ATP 之间出现负异向连接效应,从而在 10 mM MgCl 2存在的情况下,YME1L-AAA+ 对 ATP 的亲和力降低至~60 μM。分子动力学方法通过揭示 YME1L-AAA+ 的条件依赖性构象群来实现结构合理化。总而言之,这些数据表明了一个初步模型,其中 YME1L 调节其对核苷酸的亲和力,以稳定对抗此类应激条件固有的降解或不稳定性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号