当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An Allosteric Binding Site on Sortilin Regulates the Trafficking of VLDL, PCSK9, and LDLR in Hepatocytes
Biochemistry ( IF 2.9 ) Pub Date : 2020-11-05 , DOI: 10.1021/acs.biochem.0c00741 Robert P Sparks 1 , Andres S Arango 2 , Jermaine L Jenkins 3 , Wayne C Guida 4 , Emad Tajkhorshid 1, 2, 5 , Charles E Sparks 6 , Janet D Sparks 6 , Rutilio A Fratti 1, 2
Biochemistry ( IF 2.9 ) Pub Date : 2020-11-05 , DOI: 10.1021/acs.biochem.0c00741 Robert P Sparks 1 , Andres S Arango 2 , Jermaine L Jenkins 3 , Wayne C Guida 4 , Emad Tajkhorshid 1, 2, 5 , Charles E Sparks 6 , Janet D Sparks 6 , Rutilio A Fratti 1, 2
Affiliation
|
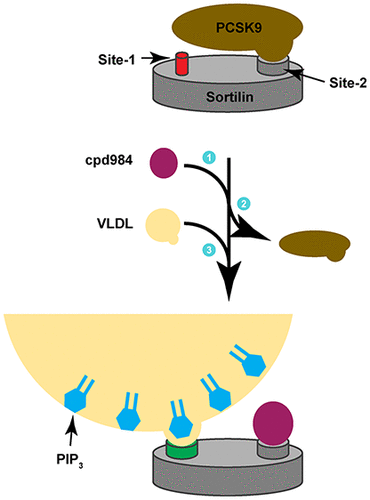
ApoB lipoproteins (apo B-Lp) are produced in hepatocytes, and their secretion requires the cargo receptor sortilin. We examined the secretion of apo B-Lp-containing very low-density lipoprotein (VLDL), an LDL progenitor. Sortilin also regulates the trafficking of the subtilase PCSK9, which when secreted binds the LDL receptor (LDLR), resulting in its endocytosis and destruction at the lysosome. We show that the site 2 binding compound (cpd984) has multiple effects in hepatocytes, including (1) enhanced Apo-Lp secretion, (2) increased cellular PCSK9 retention, and (3) augmented levels of LDLR at the plasma membrane. We postulate that cpd984 enhances apo B-Lp secretion in part through binding the lipid phosphatidylinositol 3,4,5-trisphosphate (PIP3), which is present at higher levels on circulating VLDL form fed rats relative to after fasting. We attribute the enhanced VLDL secretion to its increased binding affinity for sortilin site 1 induced by cpd984 binding site 2. This hinders PCSK9 binding and secretion, which would subsequently prevent its binding to LDLR leading to its degradation. This suggests that site 2 is an allosteric regulator of site 1 binding. This effect is not limited to VLDL, as cpd984 augments binding of the neuropeptide neurotensin (NT) to sortilin site 1. Molecular dynamics simulations demonstrate that the C-terminus of NT (Ct-NT) stably binds site 1 through an electrostatic interaction. This was bolstered by the ability of Ct-NT to disrupt lower-affinity interactions between sortilin and the site 1 ligand PIP3. Together, these data show that binding cargo at sortilin site 1 is allosterically regulated through site 2 binding, with important ramifications for cellular lipid homeostasis involving proteins such as PCSK9 and LDLR.
中文翻译:

Sortilin 上的变构结合位点调节肝细胞中 VLDL、PCSK9 和 LDLR 的运输
ApoB 脂蛋白 (apo B-Lp) 在肝细胞中产生,它们的分泌需要货物受体分拣蛋白。我们检查了含有载脂蛋白 B-Lp 的极低密度脂蛋白 (VLDL)(一种 LDL 祖细胞)的分泌。Sortilin 还调节枯草杆菌酶 PCSK9 的运输,该酶在分泌时与 LDL 受体 (LDLR) 结合,导致其在溶酶体中的内吞作用和破坏。我们表明,位点 2 结合化合物 (cpd984) 在肝细胞中具有多种作用,包括 (1) 增强 Apo-Lp 分泌,(2) 增加细胞 PCSK9 保留,以及 (3) 增加质膜上的 LDLR 水平。我们假设 cpd984 部分通过结合脂质磷脂酰肌醇 3,4,5-三磷酸 (PIP 3),相对于禁食后,其在循环 VLDL 形式喂养的大鼠中的含量更高。我们将增强的 VLDL 分泌归因于其对由 cpd984 结合位点 2 诱导的分拣蛋白位点 1 的结合亲和力增加。这阻碍了 PCSK9 的结合和分泌,随后会阻止其与 LDLR 的结合导致其降解。这表明位点 2 是位点 1 结合的变构调节剂。这种效应不仅限于 VLDL,因为 cpd984 增强了神经肽神经降压素 (NT) 与分拣蛋白位点 1 的结合。分子动力学模拟表明,NT (Ct-NT) 的 C 端通过静电相互作用稳定地结合位点 1。这得到了 Ct-NT 破坏分拣蛋白和位点 1 配体 PIP 3之间低亲和力相互作用的能力的支持. 总之,这些数据表明分拣蛋白位点 1 的结合货物通过位点 2 结合进行变构调节,对涉及蛋白质(如 PCSK9 和 LDLR)的细胞脂质稳态产生重要影响。
更新日期:2020-11-17
中文翻译:

Sortilin 上的变构结合位点调节肝细胞中 VLDL、PCSK9 和 LDLR 的运输
ApoB 脂蛋白 (apo B-Lp) 在肝细胞中产生,它们的分泌需要货物受体分拣蛋白。我们检查了含有载脂蛋白 B-Lp 的极低密度脂蛋白 (VLDL)(一种 LDL 祖细胞)的分泌。Sortilin 还调节枯草杆菌酶 PCSK9 的运输,该酶在分泌时与 LDL 受体 (LDLR) 结合,导致其在溶酶体中的内吞作用和破坏。我们表明,位点 2 结合化合物 (cpd984) 在肝细胞中具有多种作用,包括 (1) 增强 Apo-Lp 分泌,(2) 增加细胞 PCSK9 保留,以及 (3) 增加质膜上的 LDLR 水平。我们假设 cpd984 部分通过结合脂质磷脂酰肌醇 3,4,5-三磷酸 (PIP 3),相对于禁食后,其在循环 VLDL 形式喂养的大鼠中的含量更高。我们将增强的 VLDL 分泌归因于其对由 cpd984 结合位点 2 诱导的分拣蛋白位点 1 的结合亲和力增加。这阻碍了 PCSK9 的结合和分泌,随后会阻止其与 LDLR 的结合导致其降解。这表明位点 2 是位点 1 结合的变构调节剂。这种效应不仅限于 VLDL,因为 cpd984 增强了神经肽神经降压素 (NT) 与分拣蛋白位点 1 的结合。分子动力学模拟表明,NT (Ct-NT) 的 C 端通过静电相互作用稳定地结合位点 1。这得到了 Ct-NT 破坏分拣蛋白和位点 1 配体 PIP 3之间低亲和力相互作用的能力的支持. 总之,这些数据表明分拣蛋白位点 1 的结合货物通过位点 2 结合进行变构调节,对涉及蛋白质(如 PCSK9 和 LDLR)的细胞脂质稳态产生重要影响。









































 京公网安备 11010802027423号
京公网安备 11010802027423号