当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Novel Tricyclic Pyroglutamide Derivatives as Potent RORγt Inverse Agonists Identified using a Virtual Screening Approach
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2020-11-06 , DOI: 10.1021/acsmedchemlett.0c00496 Qingjie Liu 1 , Douglas G Batt 1 , Carolyn A Weigelt 1 , Shiuhang Yip 1 , Dauh-Rurng Wu 1 , Max Ruzanov 1 , John S Sack 1 , Jinhong Wang 1 , Melissa Yarde 1 , Sha Li 1 , David J Shuster 1 , Jenny H Xie 1 , Tara Sherry 1 , Mary T Obermeier 1 , Aberra Fura 1 , Kevin Stefanski 1 , Georgia Cornelius 1 , Purnima Khandelwal 1 , Joseph A Tino 1 , John E Macor 1 , Luisa Salter-Cid 1 , Rex Denton 1 , Qihong Zhao 1 , T G Murali Dhar 1
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2020-11-06 , DOI: 10.1021/acsmedchemlett.0c00496 Qingjie Liu 1 , Douglas G Batt 1 , Carolyn A Weigelt 1 , Shiuhang Yip 1 , Dauh-Rurng Wu 1 , Max Ruzanov 1 , John S Sack 1 , Jinhong Wang 1 , Melissa Yarde 1 , Sha Li 1 , David J Shuster 1 , Jenny H Xie 1 , Tara Sherry 1 , Mary T Obermeier 1 , Aberra Fura 1 , Kevin Stefanski 1 , Georgia Cornelius 1 , Purnima Khandelwal 1 , Joseph A Tino 1 , John E Macor 1 , Luisa Salter-Cid 1 , Rex Denton 1 , Qihong Zhao 1 , T G Murali Dhar 1
Affiliation
|
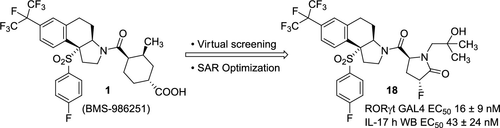
Employing a virtual screening approach, we identified the pyroglutamide moiety as a nonacid replacement for the cyclohexanecarboxylic acid group which, when coupled to our previously reported conformationally locked tricyclic core, provided potent and selective RORγt inverse agonists. Structure–activity relationship optimization of the pyroglutamide moiety led to the identification of compound 18 as a potent and selective RORγt inverse agonist, albeit with poor aqueous solubility. We took advantage of the tertiary carbinol group in 18 to synthesize a phosphate prodrug, which provided good solubility, excellent exposures in mouse PK studies, and significant efficacy in a mouse model of psoriasis.
中文翻译:

使用虚拟筛选方法鉴定作为有效 RORγt 反向激动剂的新型三环焦谷酰胺衍生物
采用虚拟筛选方法,我们将焦谷酰胺部分鉴定为环己烷羧酸基团的非酸替代物,当与我们之前报道的构象锁定三环核心结合时,提供有效和选择性的 RORγt 反向激动剂。焦谷酰胺部分的构效关系优化导致化合物18被鉴定为有效且选择性的 RORγt 反向激动剂,尽管水溶性较差。我们利用18中的叔甲醇基团合成了一种磷酸盐前药,该前药具有良好的溶解性,在小鼠 PK 研究中具有出色的暴露性,并且在银屑病小鼠模型中具有显着疗效。
更新日期:2020-12-10
中文翻译:

使用虚拟筛选方法鉴定作为有效 RORγt 反向激动剂的新型三环焦谷酰胺衍生物
采用虚拟筛选方法,我们将焦谷酰胺部分鉴定为环己烷羧酸基团的非酸替代物,当与我们之前报道的构象锁定三环核心结合时,提供有效和选择性的 RORγt 反向激动剂。焦谷酰胺部分的构效关系优化导致化合物18被鉴定为有效且选择性的 RORγt 反向激动剂,尽管水溶性较差。我们利用18中的叔甲醇基团合成了一种磷酸盐前药,该前药具有良好的溶解性,在小鼠 PK 研究中具有出色的暴露性,并且在银屑病小鼠模型中具有显着疗效。



























 京公网安备 11010802027423号
京公网安备 11010802027423号