Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of Molecular Complexity through Nitrene-Transfer Reactions Catalyzed by Copper and Silver Scorpionate Complexes
Synlett ( IF 1.7 ) Pub Date : 2020-11-05 , DOI: 10.1055/s-0040-1706534 M. Mar Díaz-Requejo 1 , Pedro J. Pérez 1 , Manuel R. Rodríguez
Synlett ( IF 1.7 ) Pub Date : 2020-11-05 , DOI: 10.1055/s-0040-1706534 M. Mar Díaz-Requejo 1 , Pedro J. Pérez 1 , Manuel R. Rodríguez
Affiliation
|
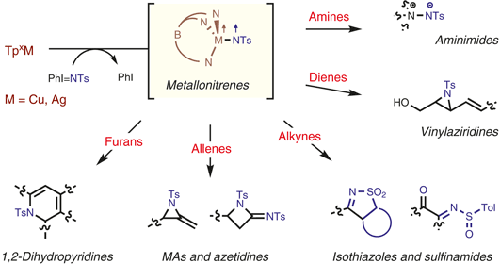
For years, metal–nitrene-transfer reactions have been mainly directed toward C=C bond aziridination or C–H bond amidation reactions. In the last decade, multiple efforts have been made to develop new synthetic approaches to expand the applicability of this powerful tool, resulting in the synthesis of complex structures from relatively simple starting materials. Here, we present our contributions to the area with several novel and, to some degree, unexpected transformations developed by our research group. The catalysts for these nitrene-transfer reactions are copper or silver complexes bearing hydrotrispyrazolyl (scorpionate) ligands. These transformations have contributed to an expansion of the scope of organic compounds accessible by nitrene-transfer reactions and to an understanding of the reactivity of metallonitrene species toward the oxidation of less-explored organic substrates. 1 Introduction 2 Synthesis of 1,2-Dihydropyridines from Furans 3 Regio- and Stereoselective Aziridination of Dienes 4 Chemoselective Synthesis of Aminimides from Amines 5 Synthesis of Sulfinamides and Isothiazoles from Alkynes 6 Formation of Azetidines and Methylene Aziridines from Allenes 7 Conclusion
中文翻译:

通过铜和蝎酸银配合物催化的氮烯转移反应发展分子复杂性
多年来,金属-氮烯转移反应主要针对 C=C 键氮丙啶化或 C-H 键酰胺化反应。在过去的十年中,已经做出了多项努力来开发新的合成方法来扩展这一强大工具的适用性,从而从相对简单的起始材料合成复杂的结构。在这里,我们通过我们的研究小组开发的几项新颖且在某种程度上出乎意料的转变来展示我们对该领域的贡献。这些氮烯转移反应的催化剂是带有氢三吡唑基(蝎形)配体的铜或银配合物。这些转变有助于扩大可通过氮烯转移反应获得的有机化合物的范围,并有助于了解金属氮烯物种对较少探索的有机底物氧化的反应性。1 引言 2 从呋喃合成 1,2-二氢吡啶 3 二烯的区域选择性和立体选择性氮丙啶化 4 从胺化学选择性合成氨基酰亚胺 5 从炔烃合成亚磺酰胺和异噻唑 6 从亚甲基氮丙啶形成氮杂环丁烷和亚甲基氮丙啶
更新日期:2020-11-05
中文翻译:

通过铜和蝎酸银配合物催化的氮烯转移反应发展分子复杂性
多年来,金属-氮烯转移反应主要针对 C=C 键氮丙啶化或 C-H 键酰胺化反应。在过去的十年中,已经做出了多项努力来开发新的合成方法来扩展这一强大工具的适用性,从而从相对简单的起始材料合成复杂的结构。在这里,我们通过我们的研究小组开发的几项新颖且在某种程度上出乎意料的转变来展示我们对该领域的贡献。这些氮烯转移反应的催化剂是带有氢三吡唑基(蝎形)配体的铜或银配合物。这些转变有助于扩大可通过氮烯转移反应获得的有机化合物的范围,并有助于了解金属氮烯物种对较少探索的有机底物氧化的反应性。1 引言 2 从呋喃合成 1,2-二氢吡啶 3 二烯的区域选择性和立体选择性氮丙啶化 4 从胺化学选择性合成氨基酰亚胺 5 从炔烃合成亚磺酰胺和异噻唑 6 从亚甲基氮丙啶形成氮杂环丁烷和亚甲基氮丙啶











































 京公网安备 11010802027423号
京公网安备 11010802027423号