当前位置:
X-MOL 学术
›
J. Neurochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeting transthyretin ‐ Mechanism‐based treatment approaches and future perspectives in hereditary amyloidosis
Journal of Neurochemistry ( IF 4.2 ) Pub Date : 2020-11-06 , DOI: 10.1111/jnc.15233 Maike F Dohrn 1, 2 , Sandra Ihne 3, 4, 5 , Ute Hegenbart 6 , Jessica Medina 2 , Stephan L Züchner 2 , Teresa Coelho 7, 8 , Katrin Hahn 9, 10
Journal of Neurochemistry ( IF 4.2 ) Pub Date : 2020-11-06 , DOI: 10.1111/jnc.15233 Maike F Dohrn 1, 2 , Sandra Ihne 3, 4, 5 , Ute Hegenbart 6 , Jessica Medina 2 , Stephan L Züchner 2 , Teresa Coelho 7, 8 , Katrin Hahn 9, 10
Affiliation
|
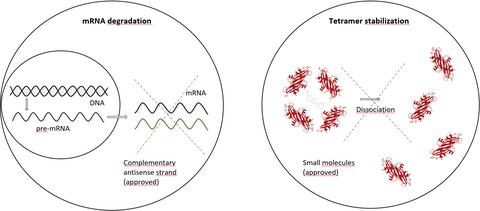
The liver‐derived, circulating transport protein transthyretin (TTR) is the cause of systemic hereditary (ATTRv) and wild‐type (ATTRwt) amyloidosis. TTR stabilization and knockdown are approved therapies to mitigate the otherwise lethal disease course. To date, the variety in phenotypic penetrance is not fully understood. This systematic review summarizes the current literature on TTR pathophysiology with its therapeutic implications. Tetramer dissociation is the rate‐limiting step of amyloidogenesis. Besides destabilizing TTR mutations, other genetic (RBP4, APCS, AR, ATX2, C1q, C3) and external (extracellular matrix, Schwann cell interaction) factors influence the type of onset and organ tropism. The approved small molecule tafamidis stabilizes the tetramer and significantly decelerates the clinical course. By sequence‐specific mRNA knockdown, the approved small interfering RNA (siRNA) patisiran and antisense oligonucleotide (ASO) inotersen both significantly reduce plasma TTR levels and improve neuropathy and quality of life compared to placebo. With enhanced hepatic targeting capabilities, GalNac‐conjugated siRNA and ASOs have recently entered phase III clinical trials. Bivalent TTR stabilizers occupy both binding groves in vitro, but have not been tested in trials so far. Tolcapone is another stabilizer with the potential to cross the blood–brain barrier, but its half‐life is short and liver failure a potential side effect. Amyloid‐directed antibodies and substances like doxycycline aim at reducing the amyloid load, however, none of the yet developed antibodies has successfully passed clinical trials. ATTR‐amyloidosis has become a model disease for pathophysiology‐based treatment. Further understanding of disease mechanisms will help to overcome the remaining limitations, including application burden, side effects, and blood–brain barrier permeability.
中文翻译:

靶向运甲状腺素蛋白-遗传性淀粉样变性病的基于机制的治疗方法和未来展望
肝脏衍生的循环转运蛋白运甲状腺素蛋白(TTR)是系统性遗传(ATTRv)和野生型(ATTRwt)淀粉样变性的原因。TTR稳定和敲除已被批准用于缓解原本致命的疾病进程。迄今为止,表型外显率的变化还没有被完全理解。该系统综述总结了有关TTR病理生理学的最新文献及其治疗意义。四聚体解离是淀粉样蛋白生成的限速步骤。除了破坏TTR突变之外,其他遗传因素(RBP4,APCS,AR,ATX2,C1q,C3)和外部因素(细胞外基质,雪旺细胞相互作用)会影响发病类型和器官嗜性。批准的小分子他法米定可稳定四聚体并显着降低临床进程。与安慰剂相比,通过序列特异性mRNA敲低,已批准的小分子干扰RNA(siRNA)patisiran和反义寡核苷酸(ASO)均能显着降低血浆TTR水平并改善神经病变和生活质量。通过增强的肝靶向能力,GalNac偶联的siRNA和ASOs最近进入了III期临床试验。二价TTR稳定剂在体外占据了两个结合区,但迄今为止尚未在试验中进行测试。托卡朋是另一种可能跨越血脑屏障的稳定剂,但是它的半衰期很短,肝功能衰竭是潜在的副作用。淀粉样蛋白定向抗体和强力霉素等物质旨在减少淀粉样蛋白负荷,但是,尚未开发的抗体均未成功通过临床试验。ATTR淀粉样变性病已成为基于病理生理学治疗的典型疾病。对疾病机制的进一步了解将有助于克服剩余的局限性,包括应用负担,副作用和血脑屏障通透性。
更新日期:2020-11-06
中文翻译:

靶向运甲状腺素蛋白-遗传性淀粉样变性病的基于机制的治疗方法和未来展望
肝脏衍生的循环转运蛋白运甲状腺素蛋白(TTR)是系统性遗传(ATTRv)和野生型(ATTRwt)淀粉样变性的原因。TTR稳定和敲除已被批准用于缓解原本致命的疾病进程。迄今为止,表型外显率的变化还没有被完全理解。该系统综述总结了有关TTR病理生理学的最新文献及其治疗意义。四聚体解离是淀粉样蛋白生成的限速步骤。除了破坏TTR突变之外,其他遗传因素(RBP4,APCS,AR,ATX2,C1q,C3)和外部因素(细胞外基质,雪旺细胞相互作用)会影响发病类型和器官嗜性。批准的小分子他法米定可稳定四聚体并显着降低临床进程。与安慰剂相比,通过序列特异性mRNA敲低,已批准的小分子干扰RNA(siRNA)patisiran和反义寡核苷酸(ASO)均能显着降低血浆TTR水平并改善神经病变和生活质量。通过增强的肝靶向能力,GalNac偶联的siRNA和ASOs最近进入了III期临床试验。二价TTR稳定剂在体外占据了两个结合区,但迄今为止尚未在试验中进行测试。托卡朋是另一种可能跨越血脑屏障的稳定剂,但是它的半衰期很短,肝功能衰竭是潜在的副作用。淀粉样蛋白定向抗体和强力霉素等物质旨在减少淀粉样蛋白负荷,但是,尚未开发的抗体均未成功通过临床试验。ATTR淀粉样变性病已成为基于病理生理学治疗的典型疾病。对疾病机制的进一步了解将有助于克服剩余的局限性,包括应用负担,副作用和血脑屏障通透性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号