当前位置:
X-MOL 学术
›
Surf. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical study of the oxygen adsorption energy for the supported Pt cluster, focused on the electronic metal-support interaction
Surface Science ( IF 2.1 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.susc.2020.121747 Kazuya Miura , Fumikazu Kimata , Ryo Watanabe , Choji Fukuhara
Surface Science ( IF 2.1 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.susc.2020.121747 Kazuya Miura , Fumikazu Kimata , Ryo Watanabe , Choji Fukuhara
|
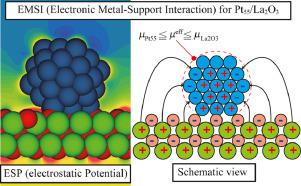
Abstract This study analyzed the icosahedral Pt55 cluster on the surface of La2O3 by density functional theory (DFT) calculations. The oxygen adsorption energy (ΔE) for the supported Pt55 cluster was different from the ΔE value for the Pt55 cluster without oxide supports. Moreover, the ΔE values for the supported Pt55 cluster depend on adsorption sites. This study applied the idea of Electronic Metal-Support Interaction (EMSI), and proposed the concept of “effective chemical potential (μeff)”. According to DFT calculation, the μeff values of supported Pt55 cluster were the intermediate values between the chemical potential values of Pt55 without oxide support (μPt55) and La2O3 (μLa2O3) (i.e. μPt55
中文翻译:

负载型 Pt 簇的氧吸附能的理论研究,侧重于电子金属-载体相互作用
摘要 本研究通过密度泛函理论(DFT) 计算分析了La2O3 表面的二十面体Pt55 簇。负载的 Pt55 簇的氧吸附能 (ΔE) 与没有氧化物载体的 Pt55 簇的 ΔE 值不同。此外,支持的 Pt55 簇的 ΔE 值取决于吸附位点。本研究应用电子金属-载体相互作用(EMSI)的思想,提出了“有效化学势(μeff)”的概念。根据 DFT 计算,支持的 Pt55 簇的 μeff 值是无氧化物支持的 Pt55(μPt55)和 La2O3(μLa2O3)(即 μPt55)化学势值之间的中间值
更新日期:2021-02-01
中文翻译:

负载型 Pt 簇的氧吸附能的理论研究,侧重于电子金属-载体相互作用
摘要 本研究通过密度泛函理论(DFT) 计算分析了La2O3 表面的二十面体Pt55 簇。负载的 Pt55 簇的氧吸附能 (ΔE) 与没有氧化物载体的 Pt55 簇的 ΔE 值不同。此外,支持的 Pt55 簇的 ΔE 值取决于吸附位点。本研究应用电子金属-载体相互作用(EMSI)的思想,提出了“有效化学势(μeff)”的概念。根据 DFT 计算,支持的 Pt55 簇的 μeff 值是无氧化物支持的 Pt55(μPt55)和 La2O3(μLa2O3)(即 μPt55)化学势值之间的中间值











































 京公网安备 11010802027423号
京公网安备 11010802027423号