Journal of Energy Chemistry ( IF 14.0 ) Pub Date : 2020-11-06 , DOI: 10.1016/j.jechem.2020.10.041 Zhe-Fan Wang , Zonglin Yi , Aziz Ahmad , Lijing Xie , Jing-Peng Chen , Qingqiang Kong , Fangyuan Su , Da-Wei Wang , Cheng-Meng Chen
|
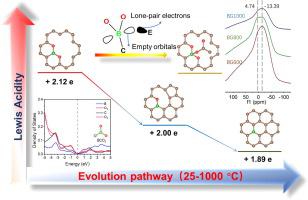
The incorporation of boron into carbon material can significantly enhance its capacity performances. However, the origin of the promotion effect of boron doping on electrochemical performances is still unclear, in part due to the inadequate exposure of boron configurations resulting from the complexity of traditional carbon materials. To overcome this issue, herein, a series of boron-doped graphene with highly-exposed boron configurations are prepared by tuning annealing temperature. Then the correlation between boron configurations and the electrochemical performances is investigated. The combination of density-functional theory (DFT) computation and NH3-TPD/Py-FTIR indicates that the BCO2 configuration formed on the surface of graphene is easier to accept lone-pair electrons than BC2O and BC3 configurations due to the stronger Lewis acidity. Such an electronic structure can effectively reduce the number of unstable electron donors and stabilize the electrochemical interface, which is proved by NMR, and critical for improving the electrochemical performances. Further experiments confirm that the optimized BG800 with the largest amount of BCO2 configuration presents ultralow leak current, improved cyclic stability, and better rate performance in SBPBF4/PC. This work would provide an insight into the design of high-performance boron-doped carbon materials towards energy storage.
中文翻译:

DFT与实验相结合:通过硼路易斯酸稳定电化学界面
将硼掺入碳材料中可以显着提高其容量性能。然而,硼掺杂对电化学性能的促进作用的起源仍不清楚,部分原因是由于传统碳材料的复杂性导致硼构型暴露不足。为了克服这个问题,本文中,通过调节退火温度来制备一系列具有高度暴露的硼构型的掺杂硼的石墨烯。然后研究了硼构型与电化学性能之间的相关性。密度泛函理论(DFT)计算和NH 3 -TPD / Py-FTIR的结合表明,石墨烯表面形成的BCO 2构型比BC更容易接受孤对电子由于路易斯酸较强,因此2 O和BC 3构型。这样的电子结构可以有效地减少不稳定的电子给体的数量并稳定电化学界面,这已通过NMR证实,这对于改善电化学性能至关重要。进一步的实验证实,具有最大数量BCO 2配置的优化BG800在SBPBF 4 / PC中表现出超低的泄漏电流,改善的循环稳定性和更好的速率性能。这项工作将为高性能的掺硼碳材料在储能方面的设计提供一个见识。











































 京公网安备 11010802027423号
京公网安备 11010802027423号