当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of hydroxyl-containing oxindoles and 3,4-dihydroquinolin-2-ones through oxone-mediated cascade arylhydroxylation of activated alkenes
Green Chemistry ( IF 9.3 ) Pub Date : 2020-10-21 , DOI: 10.1039/d0gc02205e Ming-Zhong Zhang 1, 2, 3, 4 , Long Liu 4, 5, 6, 7, 8 , Quan Gou 1, 2, 3, 4 , Qi Wang 1, 2, 3, 4 , Yi Li 1, 2, 3, 4 , Wan-Ting Li 1, 2, 3, 4 , Fei Luo 1, 2, 3, 4 , Min Yuan 1, 2, 3, 4 , Tieqiao Chen 4, 5, 6, 7, 8 , Wei-Min He 4, 9, 10, 11
Green Chemistry ( IF 9.3 ) Pub Date : 2020-10-21 , DOI: 10.1039/d0gc02205e Ming-Zhong Zhang 1, 2, 3, 4 , Long Liu 4, 5, 6, 7, 8 , Quan Gou 1, 2, 3, 4 , Qi Wang 1, 2, 3, 4 , Yi Li 1, 2, 3, 4 , Wan-Ting Li 1, 2, 3, 4 , Fei Luo 1, 2, 3, 4 , Min Yuan 1, 2, 3, 4 , Tieqiao Chen 4, 5, 6, 7, 8 , Wei-Min He 4, 9, 10, 11
Affiliation
|
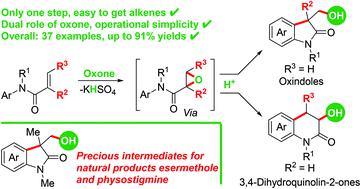
Hydroxyl-containing compounds are highly value-added organic molecules, and the establishment of novel methodologies for their elaboration is a long-standing challenge in organic synthesis. Here the first oxone-mediated direct arylhydroxylation of activated alkenes was developed for the synthesis of valuable hydroxyl-containing oxindoles and 3,4-dihydroquinolin-2-ones. The products were controlled by adjusting the structure of the starting alkenes. Moreover, the reaction was performed under simple conditions without any external additives or catalysts. Primary mechanistic studies showed that this reaction was a tandem process involving epoxidation and subsequent Friedel–Crafts alkylation, and oxone played a dual role (both the oxidant and proton source) in this process.
中文翻译:

通过酮介导的活化烯烃的级联芳基羟基化反应合成含羟基的羟吲哚和3,4-二氢喹啉-2-酮
含羟基的化合物是高附加值的有机分子,建立新颖的加工方法是有机合成中的长期挑战。在这里,首次环氧酮介导的活化烯烃的直接芳基羟基化反应被用于合成有价值的含羟基的吲哚和3,4-二氢喹啉-2-酮。通过调节起始烯烃的结构来控制产物。而且,反应在没有任何外部添加剂或催化剂的简单条件下进行。初步的机理研究表明,该反应是一个串联过程,涉及环氧化反应和随后的Friedel-Crafts烷基化反应,并且在此过程中,oxone起着双重作用(氧化剂和质子源)。
更新日期:2020-11-05
中文翻译:

通过酮介导的活化烯烃的级联芳基羟基化反应合成含羟基的羟吲哚和3,4-二氢喹啉-2-酮
含羟基的化合物是高附加值的有机分子,建立新颖的加工方法是有机合成中的长期挑战。在这里,首次环氧酮介导的活化烯烃的直接芳基羟基化反应被用于合成有价值的含羟基的吲哚和3,4-二氢喹啉-2-酮。通过调节起始烯烃的结构来控制产物。而且,反应在没有任何外部添加剂或催化剂的简单条件下进行。初步的机理研究表明,该反应是一个串联过程,涉及环氧化反应和随后的Friedel-Crafts烷基化反应,并且在此过程中,oxone起着双重作用(氧化剂和质子源)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号