当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Combinatorial miRNA-34a replenishment and irinotecan delivery via auto-fluorescent polymeric hybrid micelles for synchronous colorectal cancer theranostics
Biomaterials Science ( IF 5.8 ) Pub Date : 2020-10-23 , DOI: 10.1039/d0bm01579b Yunhao Li 1 , Fan Jia , Xiongwei Deng , Xuan Wang , Jianqing Lu , Leihou Shao , Xinyue Cui , Zian Pan , Yan Wu
Biomaterials Science ( IF 5.8 ) Pub Date : 2020-10-23 , DOI: 10.1039/d0bm01579b Yunhao Li 1 , Fan Jia , Xiongwei Deng , Xuan Wang , Jianqing Lu , Leihou Shao , Xinyue Cui , Zian Pan , Yan Wu
Affiliation
|
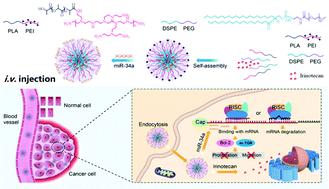
The synergistic combination of microRNA (miRNA) modulation and chemotherapy has emerged as an effective strategy to combat cancer. Irinotecan (IRI) is a potent antitumor chemotherapeutic in clinical practice and has been used for treating various malignant tumors, including colorectal cancer (CRC). However, IRI is not effective for advanced CRC or metastatic behavior. Herein, novel polymeric hybrid micelles were engineered based on two different amphiphilic copolymers, polyethyleneimine-poly(D,L-lactide) (PEI-PLA) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy (polyethyleneglycol) (DSPE-PEG), in which IRI and a tumor suppressive microRNA-34a (miR-34a) gene were efficiently co-loaded (MINPs) to achieve a chemo-miRNA combination therapy against CRC. MINPs were successfully constructed by two-step film dispersion and electrostatic interaction methods. IRI and miR-34a could be efficaciously encapsulated as MINPs and transferred to CRC cells. After encapsulation, MINPs would then upregulate miR-34a expression and regulate miR-34a-related downstream genes, which in turn led to enhanced cell cytotoxicity and apoptosis ratios. MINPs presented an excitation-dependent multi-wavelength emission feature due to the intrinstic fluorescence properties of PEI-PLA and could be utilized for in vitro/vivo imaging. According to the in vivo experimental results, MINPs possess the great characteristic of accumulating in situ in a tumor site and lightening it after intravenous administration. Furthermore, MINPs presented extraordinary antitumor efficacy owing to the combined therapy effects of IRI and miR-34a with good biocompability. Overall, our findings validated MINPs-mediated miR-34a replenishment and IRI co-delivery to serve as an effective theranostic platform and provided an innovative horizon for combining chemo-gene therapy against CRC.
中文翻译:

通过自发荧光聚合物杂合胶束补充 miRNA-34a 和伊立替康联合递送用于同步结直肠癌治疗
microRNA (miRNA) 调节和化学疗法的协同组合已成为对抗癌症的有效策略。伊立替康(IRI)在临床实践中是一种有效的抗肿瘤化疗药物,已被用于治疗多种恶性肿瘤,包括结直肠癌(CRC)。然而,IRI 对晚期 CRC 或转移行为无效。在此,基于两种不同的两亲共聚物聚乙烯亚胺-聚(D,L-丙交酯)(PEI-PLA)和 1,2-二硬脂酰-sn-甘油-3-磷酸乙醇胺-N设计了新型聚合物杂化胶束。-[甲氧基(聚乙二醇)(DSPE-PEG),其中IRI和肿瘤抑制性microRNA-34a(miR-34a)基因被有效地共同加载(MINPs)以实现针对CRC的化学-miRNA联合疗法。通过两步膜分散和静电相互作用方法成功构建了MINPs。IRI 和 miR-34a 可以有效地封装为 MINP 并转移到 CRC 细胞中。封装后,MINPs 会上调 miR-34a 的表达并调节 miR-34a 相关的下游基因,从而提高细胞的细胞毒性和凋亡率。由于 PEI-PLA 的固有荧光特性,MINPs 呈现出一种依赖于激发的多波长发射特征,可用于体外/体内成像。根据体内实验结果表明,MINPs具有在肿瘤部位原位积累和静脉给药后减轻的特点。此外,由于IRI和miR-34a的联合治疗效果具有良好的生物相容性,MINPs表现出非凡的抗肿瘤功效。总体而言,我们的研究结果验证了 MINPs 介导的 miR-34a 补充和 IRI 共同递送可作为有效的治疗诊断平台,并为将化学基因治疗与 CRC 相结合提供了创新视野。
更新日期:2020-11-05
中文翻译:

通过自发荧光聚合物杂合胶束补充 miRNA-34a 和伊立替康联合递送用于同步结直肠癌治疗
microRNA (miRNA) 调节和化学疗法的协同组合已成为对抗癌症的有效策略。伊立替康(IRI)在临床实践中是一种有效的抗肿瘤化疗药物,已被用于治疗多种恶性肿瘤,包括结直肠癌(CRC)。然而,IRI 对晚期 CRC 或转移行为无效。在此,基于两种不同的两亲共聚物聚乙烯亚胺-聚(D,L-丙交酯)(PEI-PLA)和 1,2-二硬脂酰-sn-甘油-3-磷酸乙醇胺-N设计了新型聚合物杂化胶束。-[甲氧基(聚乙二醇)(DSPE-PEG),其中IRI和肿瘤抑制性microRNA-34a(miR-34a)基因被有效地共同加载(MINPs)以实现针对CRC的化学-miRNA联合疗法。通过两步膜分散和静电相互作用方法成功构建了MINPs。IRI 和 miR-34a 可以有效地封装为 MINP 并转移到 CRC 细胞中。封装后,MINPs 会上调 miR-34a 的表达并调节 miR-34a 相关的下游基因,从而提高细胞的细胞毒性和凋亡率。由于 PEI-PLA 的固有荧光特性,MINPs 呈现出一种依赖于激发的多波长发射特征,可用于体外/体内成像。根据体内实验结果表明,MINPs具有在肿瘤部位原位积累和静脉给药后减轻的特点。此外,由于IRI和miR-34a的联合治疗效果具有良好的生物相容性,MINPs表现出非凡的抗肿瘤功效。总体而言,我们的研究结果验证了 MINPs 介导的 miR-34a 补充和 IRI 共同递送可作为有效的治疗诊断平台,并为将化学基因治疗与 CRC 相结合提供了创新视野。










































 京公网安备 11010802027423号
京公网安备 11010802027423号