当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemical Architecture of Block Copolymers Differentially Abrogate Cardiotoxicity and Maintain the Anticancer Efficacy of Doxorubicin
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-11-05 , DOI: 10.1021/acs.molpharmaceut.0c00963 Chowdhury S Abdullah 1 , Priyanka Ray 2 , Shafiul Alam 1 , Narendra Kale 2 , Richa Aishwarya 3 , Mahboob Morshed 1 , Debasmita Dutta 2 , Cathleen Hudziak 4 , Sushanta K Banerjee 5, 6 , Sanku Mallik 7 , Snigdha Banerjee 5, 6 , Md Shenuarin Bhuiyan 1, 3 , Mohiuddin Quadir 2
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-11-05 , DOI: 10.1021/acs.molpharmaceut.0c00963 Chowdhury S Abdullah 1 , Priyanka Ray 2 , Shafiul Alam 1 , Narendra Kale 2 , Richa Aishwarya 3 , Mahboob Morshed 1 , Debasmita Dutta 2 , Cathleen Hudziak 4 , Sushanta K Banerjee 5, 6 , Sanku Mallik 7 , Snigdha Banerjee 5, 6 , Md Shenuarin Bhuiyan 1, 3 , Mohiuddin Quadir 2
Affiliation
|
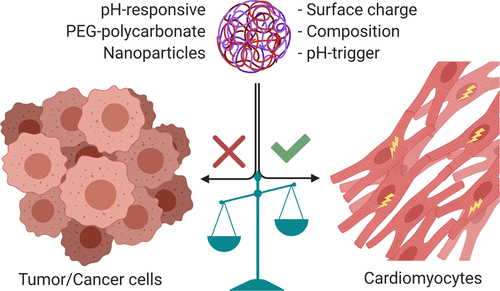
The molecular architecture of pH-responsive amphiphilic block copolymers, their self-assembly behavior to form nanoparticles (NPs), and doxorubicin (DOX)-loading technique govern the extent of DOX-induced cardiotoxicity. We observed that the choice of pH-sensitive tertiary amines, surface charge, and DOX-loading techniques within the self-assembled NPs strongly influence the release and stimulation of DOX-induced cardiotoxicity in primary cardiomyocytes. However, covalent conjugation of DOX to a pH-sensitive nanocarrier through a “conditionally unstable amide” linkage (PCPY–cDOX; PC = polycarbonate and PY = 2-pyrrolidine-1-yl-ethyl-amine) significantly reduced the cardiotoxicity of DOX in cardiomyocytes as compared to noncovalently encapsulated DOX NPs (PCPY–eDOX). When these formulations were tested for drug release in serum-containing media, the PCPY–cDOX systems showed prolonged control over drug release (for ∼72 h) at acidic pH compared to DOX-encapsulated nanocarriers, as expected. We found that DOX-encapsulated nanoformulations triggered cardiotoxicity in primary cardiomyocytes more acutely, while conjugated systems such as PCPY–cDOX prevented cardiotoxicity by disabling the nuclear entry of the drug. Using 2D and 3D (spheroid) cultures of an ER + breast cancer cell line (MCF-7) and a triple-negative breast cancer cell line (MDA-MB-231), we unravel that, similar to encapsulated systems (PCPY–eDOX-type) as reported earlier, the PCPY–cDOX system suppresses cellular proliferation in both cell lines and enhances trafficking through 3D spheroids of MDA-MB-231 cells. Collectively, our studies indicate that PCPY–cDOX is less cardiotoxic as compared to noncovalently encapsulated variants without compromising the chemotherapeutic properties of the drug. Thus, our studies suggest that the appropriate selection of the nanocarrier for DOX delivery may prove fruitful in shifting the balance between low cardiotoxicity and triggering the chemotherapeutic potency of DOX.
中文翻译:

嵌段共聚物的化学结构不同地消除心脏毒性并保持多柔比星的抗癌功效
pH 响应型两亲嵌段共聚物的分子结构、它们形成纳米颗粒 (NPs) 的自组装行为和阿霉素 (DOX) 负载技术决定了 DOX 诱导的心脏毒性的程度。我们观察到,在自组装 NP 中选择 pH 敏感的叔胺、表面电荷和 DOX 加载技术强烈影响原代心肌细胞中 DOX 诱导的心脏毒性的释放和刺激。然而,DOX 通过“条件不稳定的酰胺”键(PCPY-cDOX;PC = 聚碳酸酯和 PY = 2-吡咯烷-1-基-乙胺)与 pH 敏感纳米载体共价共轭显着降低了 DOX 在心肌细胞与非共价封装的 DOX NPs (PCPY-eDOX) 相比。当这些制剂在含血清介质中进行药物释放测试时,正如预期的那样,与 DOX 封装的纳米载体相比,PCPY-cDOX 系统在酸性 pH 下显示出对药物释放的延长控制(约 72 小时)。我们发现,包裹 DOX 的纳米制剂更强烈地触发了原代心肌细胞的心脏毒性,而诸如 PCPY-cDOX 的共轭系统通过禁用药物的核进入来防止心脏毒性。使用 ER + 乳腺癌细胞系 (MCF-7) 和三阴性乳腺癌细胞系 (MDA-MB-231) 的 2D 和 3D(球体)培养物,我们揭示了这一点,类似于封装系统 (PCPY–eDOX) -type) 如前所述,PCPY-cDOX 系统抑制两种细胞系中的细胞增殖并增强通过 MDA-MB-231 细胞的 3D 球体的运输。集体,我们的研究表明,与非共价封装的变体相比,PCPY-cDOX 的心脏毒性较小,而不会影响药物的化学治疗特性。Thus, our studies suggest that the appropriate selection of the nanocarrier for DOX delivery may prove fruitful in shifting the balance between low cardiotoxicity and triggering the chemotherapeutic potency of DOX.
更新日期:2020-12-07
中文翻译:

嵌段共聚物的化学结构不同地消除心脏毒性并保持多柔比星的抗癌功效
pH 响应型两亲嵌段共聚物的分子结构、它们形成纳米颗粒 (NPs) 的自组装行为和阿霉素 (DOX) 负载技术决定了 DOX 诱导的心脏毒性的程度。我们观察到,在自组装 NP 中选择 pH 敏感的叔胺、表面电荷和 DOX 加载技术强烈影响原代心肌细胞中 DOX 诱导的心脏毒性的释放和刺激。然而,DOX 通过“条件不稳定的酰胺”键(PCPY-cDOX;PC = 聚碳酸酯和 PY = 2-吡咯烷-1-基-乙胺)与 pH 敏感纳米载体共价共轭显着降低了 DOX 在心肌细胞与非共价封装的 DOX NPs (PCPY-eDOX) 相比。当这些制剂在含血清介质中进行药物释放测试时,正如预期的那样,与 DOX 封装的纳米载体相比,PCPY-cDOX 系统在酸性 pH 下显示出对药物释放的延长控制(约 72 小时)。我们发现,包裹 DOX 的纳米制剂更强烈地触发了原代心肌细胞的心脏毒性,而诸如 PCPY-cDOX 的共轭系统通过禁用药物的核进入来防止心脏毒性。使用 ER + 乳腺癌细胞系 (MCF-7) 和三阴性乳腺癌细胞系 (MDA-MB-231) 的 2D 和 3D(球体)培养物,我们揭示了这一点,类似于封装系统 (PCPY–eDOX) -type) 如前所述,PCPY-cDOX 系统抑制两种细胞系中的细胞增殖并增强通过 MDA-MB-231 细胞的 3D 球体的运输。集体,我们的研究表明,与非共价封装的变体相比,PCPY-cDOX 的心脏毒性较小,而不会影响药物的化学治疗特性。Thus, our studies suggest that the appropriate selection of the nanocarrier for DOX delivery may prove fruitful in shifting the balance between low cardiotoxicity and triggering the chemotherapeutic potency of DOX.











































 京公网安备 11010802027423号
京公网安备 11010802027423号