当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Denigrins and Dactylpyrroles, Arylpyrrole Alkaloids from a Dactylia sp. Marine Sponge
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-11-05 , DOI: 10.1021/acs.jnatprod.0c01103 Unwoo Kang 1 , Laura K Cartner 1, 2 , Dongdong Wang 1 , Chang-Kwon Kim 1 , Cheryl L Thomas 1, 2 , Girma M Woldemichael 1, 2 , Berkley E Gryder 3 , John F Shern 3 , Javed Khan 3 , Cristiana Castello-Branco 4 , Edward C Sherer 5 , Xiao Wang 5 , Erik L Regalado 5 , Kirk R Gustafson 1
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-11-05 , DOI: 10.1021/acs.jnatprod.0c01103 Unwoo Kang 1 , Laura K Cartner 1, 2 , Dongdong Wang 1 , Chang-Kwon Kim 1 , Cheryl L Thomas 1, 2 , Girma M Woldemichael 1, 2 , Berkley E Gryder 3 , John F Shern 3 , Javed Khan 3 , Cristiana Castello-Branco 4 , Edward C Sherer 5 , Xiao Wang 5 , Erik L Regalado 5 , Kirk R Gustafson 1
Affiliation
|
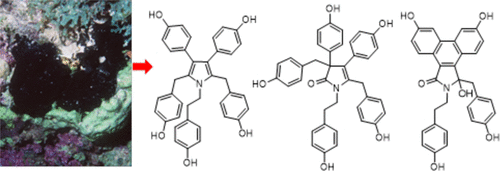
Seven new arylpyrrole alkaloids (1–7), along with four known compounds, were isolated from an extract of a Dactylia sp. nov. marine sponge, and their structures were elucidated by interpretation of NMR and MS spectroscopic data. Denigrins D–G (1–4) have highly substituted pyrrole or pyrrolone rings in their core structures, while dactylpyrroles A–C (5–7) have tricyclic phenanthrene cores. Due to the proton-deficient nature of these scaffolds, key heteronuclear correlations from 1H–15N HMBC and LR-HSQMBC NMR experiments were used in the structure assignment of denigrin D (1). Dictyodendrin F (8), a previously described co-metabolite, inhibited transcription driven by the oncogenic PAX3-FOXO1 fusion gene with an IC50 value of 13 μM.
中文翻译:

来自 Dactylia sp. 的 Denigrins 和 Dactylpyrroles、Arylpyrrole 生物碱。海洋海绵
从指藻提取物中分离出七种新的芳基吡咯生物碱 ( 1 – 7 ) 以及四种已知化合物。十一月通过核磁共振和质谱数据的解释阐明了它们的结构。 Denigrins D–G ( 1 – 4 ) 在其核心结构中具有高度取代的吡咯或吡咯酮环,而dactylpyrroles A–C ( 5 – 7 ) 则具有三环菲核心。由于这些支架的质子缺乏性质,来自1 H– 15 N HMBC 和 LR-HSQMBC NMR 实验的关键异核关联被用于 denigrin D 的结构分配 ( 1 )。 Dictyodendrin F ( 8 ) 是一种先前描述的共代谢物,可抑制由致癌 PAX3-FOXO1 融合基因驱动的转录,IC 50值为 13 μM。
更新日期:2020-11-25
中文翻译:

来自 Dactylia sp. 的 Denigrins 和 Dactylpyrroles、Arylpyrrole 生物碱。海洋海绵
从指藻提取物中分离出七种新的芳基吡咯生物碱 ( 1 – 7 ) 以及四种已知化合物。十一月通过核磁共振和质谱数据的解释阐明了它们的结构。 Denigrins D–G ( 1 – 4 ) 在其核心结构中具有高度取代的吡咯或吡咯酮环,而dactylpyrroles A–C ( 5 – 7 ) 则具有三环菲核心。由于这些支架的质子缺乏性质,来自1 H– 15 N HMBC 和 LR-HSQMBC NMR 实验的关键异核关联被用于 denigrin D 的结构分配 ( 1 )。 Dictyodendrin F ( 8 ) 是一种先前描述的共代谢物,可抑制由致癌 PAX3-FOXO1 融合基因驱动的转录,IC 50值为 13 μM。











































 京公网安备 11010802027423号
京公网安备 11010802027423号