当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Vapor–Liquid Equilibrium for the Binary of Propylene Glycol Methyl Ether Acetate + Ethyl Lactate and Propylene Glycol Methyl Ether + Ethyl Lactate at 101.3 and 20.0 kPa
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-11-05 , DOI: 10.1021/acs.jced.0c00488 Xusheng Mi 1 , Changsheng Yang 1 , Feizhong Sun 1 , Bao Li 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-11-05 , DOI: 10.1021/acs.jced.0c00488 Xusheng Mi 1 , Changsheng Yang 1 , Feizhong Sun 1 , Bao Li 1
Affiliation
|
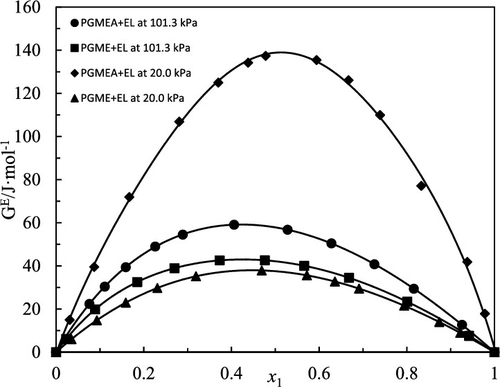
In this work, the vapor–liquid equilibrium (VLE) data of propylene glycol methyl ether acetate + ethyl lactate and propylene glycol methyl ether + ethyl lactate was determined by a modified Rose–Williams still at 101.3 and 20.0 kPa. The area test, the Van Ness test, and pure component consistency test were used to check the VLE data’s thermodynamic consistency. The nonrandom two-liquid (NRTL), universal quasi-chemical (UNIQUAC), and Wilson activity coefficient models were used for correlation of the experimental data. Consequently, the experimental data showed good correlation with the calculated data of three models and was reliable to apply to practice.
中文翻译:

丙二醇甲醚乙酸酯+乳酸乙酯和丙二醇甲醚+乳酸乙酯二元的汽液平衡在101.3和20.0 kPa
在这项工作中,丙二醇甲醚乙酸酯+乳酸乙酯和丙二醇甲醚+乳酸乙酯的气液平衡(VLE)数据是通过仍在101.3和20.0 kPa的改良Rose-Williams测定的。面积测试,Van Ness测试和纯组分一致性测试用于检查VLE数据的热力学一致性。使用非随机两液(NRTL),通用拟化学(UNIQUAC)和Wilson活度系数模型来关联实验数据。因此,实验数据与三个模型的计算数据具有良好的相关性,可以可靠地应用于实践。
更新日期:2021-01-14
中文翻译:

丙二醇甲醚乙酸酯+乳酸乙酯和丙二醇甲醚+乳酸乙酯二元的汽液平衡在101.3和20.0 kPa
在这项工作中,丙二醇甲醚乙酸酯+乳酸乙酯和丙二醇甲醚+乳酸乙酯的气液平衡(VLE)数据是通过仍在101.3和20.0 kPa的改良Rose-Williams测定的。面积测试,Van Ness测试和纯组分一致性测试用于检查VLE数据的热力学一致性。使用非随机两液(NRTL),通用拟化学(UNIQUAC)和Wilson活度系数模型来关联实验数据。因此,实验数据与三个模型的计算数据具有良好的相关性,可以可靠地应用于实践。










































 京公网安备 11010802027423号
京公网安备 11010802027423号