当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Decoupling Protein Production from Cell Growth Enhances the Site-Specific Incorporation of Noncanonical Amino Acids in E. coli
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-11-05 , DOI: 10.1021/acssynbio.0c00298 Meritxell Galindo Casas 1, 2 , Patrick Stargardt 3 , Juergen Mairhofer 3 , Birgit Wiltschi 1
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-11-05 , DOI: 10.1021/acssynbio.0c00298 Meritxell Galindo Casas 1, 2 , Patrick Stargardt 3 , Juergen Mairhofer 3 , Birgit Wiltschi 1
Affiliation
|
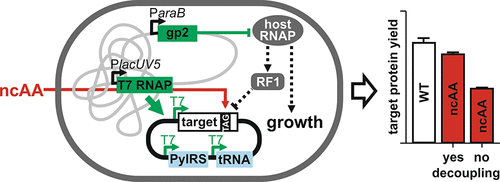
The site-specific incorporation of noncanonical amino acids (ncAAs) into proteins by amber stop codon suppression has become a routine method in academic laboratories. This approach requires an amber suppressor tRNACUA to read the amber codon and an aminoacyl-tRNA synthetase to charge the tRNACUA with the ncAA. However, a major drawback is the low yield of the mutant protein in comparison to the wild type. This effect primarily results from the competition of release factor 1 with the charged suppressor tRNACUA for the amber codon at the A-site of the ribosome. A number of laboratories have attempted to improve the incorporation efficiency of ncAAs with moderate results. We aimed at increasing the efficiency to produce high yields of ncAA-functionalized proteins in a scalable setting for industrial application. To do this, we inserted an ncAA into the enhanced green fluorescent protein and an antibody mimetic molecule using an industrial E. coli strain, which produces recombinant proteins independent of cell growth. The controlled decoupling of recombinant protein production from cell growth considerably increased the incorporation of the ncAA, producing substantially higher protein yields versus the reference E. coli strain BL21(DE3). The target proteins were expressed at high levels, and the ncAA was efficiently incorporated with excellent fidelity while the protein function was preserved.
中文翻译:

从细胞生长中分离蛋白质生产增强了非经典氨基酸在大肠杆菌中的位点特异性结合
通过琥珀终止密码子抑制将非规范氨基酸 (ncAAs) 特定部位掺入蛋白质已成为学术实验室的常规方法。这种方法需要一个琥珀抑制 tRNA CUA来读取琥珀密码子和氨酰 tRNA 合成酶来用 ncAA为 tRNA CUA充电。然而,一个主要的缺点是与野生型相比,突变蛋白的产量低。这种效应主要是由于释放因子 1 与带电抑制因子 tRNA CUA竞争为位于核糖体 A 位点的琥珀密码子。许多实验室试图以中等结果提高 ncAAs 的合并效率。我们旨在提高效率,在工业应用的可扩展环境中生产高产量的 ncAA 功能化蛋白质。为此,我们使用工业大肠杆菌菌株将 ncAA 插入增强型绿色荧光蛋白和抗体模拟分子中,该菌株可产生独立于细胞生长的重组蛋白。重组蛋白生产与细胞生长的受控解耦大大增加了 ncAA 的掺入,与参考大肠杆菌相比,产生了更高的蛋白质产量菌株 BL21(DE3)。目标蛋白以高水平表达,并且 ncAA 以出色的保真度有效地结合,同时保留了蛋白质功能。
更新日期:2020-11-21
中文翻译:

从细胞生长中分离蛋白质生产增强了非经典氨基酸在大肠杆菌中的位点特异性结合
通过琥珀终止密码子抑制将非规范氨基酸 (ncAAs) 特定部位掺入蛋白质已成为学术实验室的常规方法。这种方法需要一个琥珀抑制 tRNA CUA来读取琥珀密码子和氨酰 tRNA 合成酶来用 ncAA为 tRNA CUA充电。然而,一个主要的缺点是与野生型相比,突变蛋白的产量低。这种效应主要是由于释放因子 1 与带电抑制因子 tRNA CUA竞争为位于核糖体 A 位点的琥珀密码子。许多实验室试图以中等结果提高 ncAAs 的合并效率。我们旨在提高效率,在工业应用的可扩展环境中生产高产量的 ncAA 功能化蛋白质。为此,我们使用工业大肠杆菌菌株将 ncAA 插入增强型绿色荧光蛋白和抗体模拟分子中,该菌株可产生独立于细胞生长的重组蛋白。重组蛋白生产与细胞生长的受控解耦大大增加了 ncAA 的掺入,与参考大肠杆菌相比,产生了更高的蛋白质产量菌株 BL21(DE3)。目标蛋白以高水平表达,并且 ncAA 以出色的保真度有效地结合,同时保留了蛋白质功能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号