当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intrinsic Origin of Tau Protein Aggregation: Effects of Histidine Tautomerism on Tau267–312 Monomer
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2020-11-04 , DOI: 10.1021/acschemneuro.0c00587 Sompriya Chatterjee 1 , Abbas Salimi 1 , Jin Yong Lee 1
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2020-11-04 , DOI: 10.1021/acschemneuro.0c00587 Sompriya Chatterjee 1 , Abbas Salimi 1 , Jin Yong Lee 1
Affiliation
|
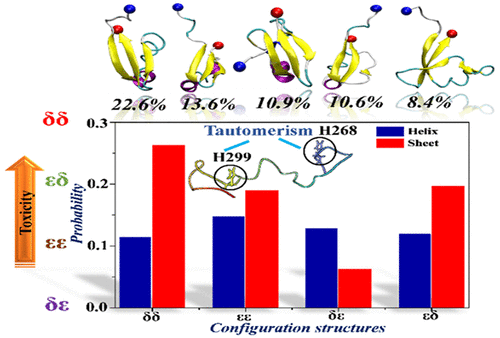
Histidine tautomerism is considered a crucial component that affects the constitutional and accumulation characteristics of the tau267–312 monomer in the neutral condition, which are connected with the pathobiology of Alzheimer’s disease (AD). Interpreting the organizational characteristics and accumulation procedure is a challenging task because two tautomeric conformations (the Nε–H or Nδ–H tautomer) can occur in the open neutral condition. In the current work, replica-exchange molecular dynamics (REMD) simulations were performed to investigate the structural properties of the tau267–312 monomer considering the histidine tautomeric effect. Based on the simulation outcomes, the histidine 268 (H268) (δ)–H299 (δ) (δδ) isomer had the highest β-sheet content with a value of 26.2%, which acquires a sheet-governing toxic conformer with the first abundant conformational state of 22.6%. In addition, δδ displayed notable antiparallel β-sheets between lysine 8 (K8)–asparagine 13 (N13) and valine 40 (V40)–tyrosine 44 (Y44) as well as between K32–H33 and V40–Y44 (β-meander supersecondary structure), indicating this tautomeric isomer may exist to stimulate tau oligomerization. Furthermore, H299 was found to play an essential role in the structural stabilization of the δδ isomer compared with H268. The present research will aid in obtaining insight into the organizational and accumulation properties of tau protein in the presence of histidine tautomerism to control AD.
中文翻译:

Tau蛋白聚集的内在起源:组氨酸互变异构对Tau 267–312单体的影响
组氨酸互变异构被认为是影响tau 267-312单体在中性条件下的组成和积累特性的重要成分,这与阿尔茨海默氏病(AD)的病理生物学有关。解释组织特征和积累过程是一项艰巨的任务,因为在开放中性条件下可能会出现两个互变异构构象(Nε- H或Nδ- H互变异构体)。在当前的工作中,进行了副本交换分子动力学(REMD)模拟以研究tau 267-312的结构特性考虑组氨酸互变异构效应的单体。根据模拟结果,组氨酸268(H268)(δ)–H299(δ)(δδ)异构体的β-片层含量最高,值为26.2%,获得了具有片层毒性的构象异构体,其中第一个丰富。构象状态为22.6%。此外,δδ在赖氨酸8(K8)–天冬酰胺13(N13)和缬氨酸40(V40)–酪氨酸44(Y44)之间以及K32–H33和V40–Y44(β曲折超中学)之间显示出显着的反平行β折叠结构),表明该互变异构体可能存在以刺激tau寡聚。此外,与H268相比,发现H299在δδ异构体的结构稳定中起着至关重要的作用。
更新日期:2020-11-18
中文翻译:

Tau蛋白聚集的内在起源:组氨酸互变异构对Tau 267–312单体的影响
组氨酸互变异构被认为是影响tau 267-312单体在中性条件下的组成和积累特性的重要成分,这与阿尔茨海默氏病(AD)的病理生物学有关。解释组织特征和积累过程是一项艰巨的任务,因为在开放中性条件下可能会出现两个互变异构构象(Nε- H或Nδ- H互变异构体)。在当前的工作中,进行了副本交换分子动力学(REMD)模拟以研究tau 267-312的结构特性考虑组氨酸互变异构效应的单体。根据模拟结果,组氨酸268(H268)(δ)–H299(δ)(δδ)异构体的β-片层含量最高,值为26.2%,获得了具有片层毒性的构象异构体,其中第一个丰富。构象状态为22.6%。此外,δδ在赖氨酸8(K8)–天冬酰胺13(N13)和缬氨酸40(V40)–酪氨酸44(Y44)之间以及K32–H33和V40–Y44(β曲折超中学)之间显示出显着的反平行β折叠结构),表明该互变异构体可能存在以刺激tau寡聚。此外,与H268相比,发现H299在δδ异构体的结构稳定中起着至关重要的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号