当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
N-Arylimide Molecular Balances: A Comprehensive Platform for Studying Aromatic Interactions in Solution
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-11-05 , DOI: 10.1021/acs.accounts.0c00519 Ping Li 1 , Erik C. Vik 2 , Ken D. Shimizu 3
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-11-05 , DOI: 10.1021/acs.accounts.0c00519 Ping Li 1 , Erik C. Vik 2 , Ken D. Shimizu 3
Affiliation
|
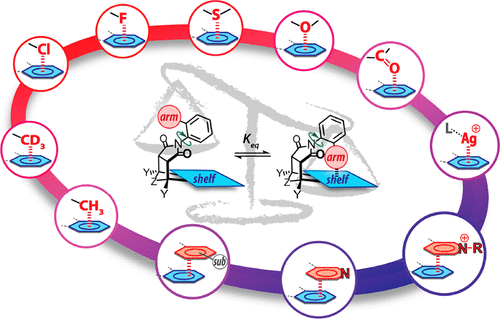
Noncovalent interactions of aromatic surfaces play a key role in many biological processes and in determining the properties and utility of synthetic materials, sensors, and catalysts. However, the study of aromatic interactions has been challenging because these interactions are usually very weak and their trends are modulated by many factors such as structural, electronic, steric, and solvent effects. Recently, N-arylimide molecular balances have emerged as highly versatile and effective platforms for studying aromatic interactions in solution. These molecular balances can accurately measure weak noncovalent interactions in solution via their influence on the folded–unfolded conformational equilibrium. The structure (i.e., size, shape, π-conjugation, and substitution) and nature (i.e., element, charge, and polarity) of the π-surfaces and interacting groups can be readily varied, enabling the study of a wide range of aromatic interactions. These include aromatic stacking, heterocyclic aromatic stacking, and alkyl−π, chalcogen−π, silver−π, halogen−π, substituent−π, and solvent−π interactions. The ability to measure a diverse array of aromatic interactions within a single model system provides a unique perspective and insights as the interaction energies, stability trends, and solvent effects for different types of interactions can be directly compared. Some broad conclusions that have emerged from this comprehensive analysis include: (1) The strongest aromatic interactions involve groups with positive charges such as pyridinium and metal ions which interact with the electrostatically negative π-face of the aromatic surface via cation−π or metal−π interactions. Attractive electrostatic interactions can also form between aromatic surfaces and groups with partial positive charges. (2) Electrostatic interactions involving aromatic surfaces can be switched from repulsive to attractive using electron-withdrawing substituents or heterocycles. These electrostatic trends appear to span many types of aromatic interactions involving a polar group interacting with a π-surface such as halogen−π, chalcogen−π, and carbonyl−π. (3) Nonpolar groups form weak but measurable stabilizing interactions with aromatic surfaces in organic solvents due to favorable dispersion and/or solvophobic effects. A good predictor of the interaction strength is provided by the change in solvent-accessible surface area. (4) Solvent effects modulate the aromatic interactions in the forms of solvophobic effects and competitive solvation, which can be modeled using solvent cohesion density and specific solvent–solute interactions.
中文翻译:

N-丙烯酰亚胺分子平衡:研究溶液中芳香相互作用的综合平台
芳族表面的非共价相互作用在许多生物过程中以及在决定合成材料,传感器和催化剂的性质和实用性方面起着关键作用。然而,芳族相互作用的研究一直具有挑战性,因为这些相互作用通常非常弱,并且它们的趋势受到许多因素的调节,例如结构,电子,空间和溶剂效应。最近,N-芳基酰亚胺分子平衡已经成为研究溶液中芳族相互作用的高度通用和有效的平台。这些分子平衡可以通过影响折叠-展开构象平衡来精确测量溶液中的弱非共价相互作用。π表面和相互作用基团的结构(即大小,形状,π共轭和取代)和性质(即元素,电荷和极性)可以很容易地改变,从而可以研究各种芳族化合物互动。这些包括芳族堆积,杂环芳族堆积以及烷基-π,硫属元素-π,银-π,卤素-π,取代基-π和溶剂-π相互作用。在单一模型系统中测量多种多样的芳香族相互作用的能力提供了独特的视角和见解,包括相互作用能,可以直接比较稳定性趋势和不同类型相互作用的溶剂效应。这项综合分析得出的一些广泛结论包括:(1)最强的芳香族相互作用涉及带有正电荷的基团,例如吡啶鎓和金属离子,它们通过阳离子-π或金属-与芳香族表面的静电负π面相互作用。 π相互作用。芳香表面和带有部分正电荷的基团之间也可能形成有吸引力的静电相互作用。(2)可以使用吸电子取代基或杂环将涉及芳族表面的静电相互作用从排斥性转换为吸引性。这些静电趋势似乎跨越了许多类型的芳香族相互作用,包括与π表面相互作用的极性基团,例如卤素-π,硫族元素-π,和羰基-π。(3)由于有利的分散和/或疏溶剂作用,非极性基团与有机溶剂中的芳族表面形成弱但可测量的稳定相互作用。溶剂可及表面积的变化可以很好地预测相互作用强度。(4)溶剂效应以疏溶剂效应和竞争性溶剂化的形式调节芳族相互作用,可使用溶剂内聚密度和特定的溶剂-溶质相互作用来模拟。
更新日期:2020-11-17
中文翻译:

N-丙烯酰亚胺分子平衡:研究溶液中芳香相互作用的综合平台
芳族表面的非共价相互作用在许多生物过程中以及在决定合成材料,传感器和催化剂的性质和实用性方面起着关键作用。然而,芳族相互作用的研究一直具有挑战性,因为这些相互作用通常非常弱,并且它们的趋势受到许多因素的调节,例如结构,电子,空间和溶剂效应。最近,N-芳基酰亚胺分子平衡已经成为研究溶液中芳族相互作用的高度通用和有效的平台。这些分子平衡可以通过影响折叠-展开构象平衡来精确测量溶液中的弱非共价相互作用。π表面和相互作用基团的结构(即大小,形状,π共轭和取代)和性质(即元素,电荷和极性)可以很容易地改变,从而可以研究各种芳族化合物互动。这些包括芳族堆积,杂环芳族堆积以及烷基-π,硫属元素-π,银-π,卤素-π,取代基-π和溶剂-π相互作用。在单一模型系统中测量多种多样的芳香族相互作用的能力提供了独特的视角和见解,包括相互作用能,可以直接比较稳定性趋势和不同类型相互作用的溶剂效应。这项综合分析得出的一些广泛结论包括:(1)最强的芳香族相互作用涉及带有正电荷的基团,例如吡啶鎓和金属离子,它们通过阳离子-π或金属-与芳香族表面的静电负π面相互作用。 π相互作用。芳香表面和带有部分正电荷的基团之间也可能形成有吸引力的静电相互作用。(2)可以使用吸电子取代基或杂环将涉及芳族表面的静电相互作用从排斥性转换为吸引性。这些静电趋势似乎跨越了许多类型的芳香族相互作用,包括与π表面相互作用的极性基团,例如卤素-π,硫族元素-π,和羰基-π。(3)由于有利的分散和/或疏溶剂作用,非极性基团与有机溶剂中的芳族表面形成弱但可测量的稳定相互作用。溶剂可及表面积的变化可以很好地预测相互作用强度。(4)溶剂效应以疏溶剂效应和竞争性溶剂化的形式调节芳族相互作用,可使用溶剂内聚密度和特定的溶剂-溶质相互作用来模拟。











































 京公网安备 11010802027423号
京公网安备 11010802027423号