当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Halogen Bond‐Catalyzed Friedel−Crafts Reactions of Furans Using a 2,2’‐Bipyridine‐Based Catalyst
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-11-04 , DOI: 10.1002/adsc.202001019 Huimiao Zhang 1 , Patrick H. Toy 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-11-04 , DOI: 10.1002/adsc.202001019 Huimiao Zhang 1 , Patrick H. Toy 1
Affiliation
|
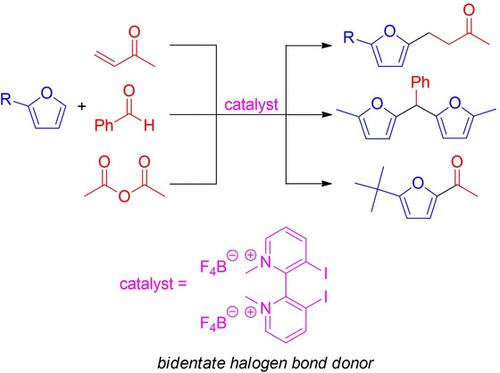
A halogen bond donor based on a 2,2’‐bipyridine framework has been synthesized, and used to catalyze Friedel−Crafts reactions of furans. Electrophiles used successfully in these reactions included various enones, an aldehyde, and a carboxylic acid anhydride. The yields of the reactions were generally good using a moderate catalyst loading (0.025 or 0.1 equiv.) at a relatively low temperature (room temp. or 50 °C) in acetonitrile. The catalyst used was designed with a biaryl scaffold so that if it indeed proved to be an efficient halogen bond donor organocatalyst, an enantioenriched version of it could potentially serve as a stereoselective catalyst.
中文翻译:

使用2,2'-联吡啶为基础的催化剂进行呋喃的卤素键催化的Friedel-Crafts反应
基于2,2'-联吡啶骨架的卤素键供体已经合成,并用于催化呋喃的Friedel-Crafts反应。在这些反应中成功使用的亲电试剂包括各种烯酮,醛和羧酸酐。在相对较低的温度(室温或50℃)下,在乙腈中使用适度的催化剂负载量(0.025或0.1当量),反应的收率通常很高。使用的催化剂设计有联芳基支架,因此,如果确实证明它是有效的卤素键供体有机催化剂,则其对映体富集的形式可能会用作立体选择性催化剂。
更新日期:2021-01-05
中文翻译:

使用2,2'-联吡啶为基础的催化剂进行呋喃的卤素键催化的Friedel-Crafts反应
基于2,2'-联吡啶骨架的卤素键供体已经合成,并用于催化呋喃的Friedel-Crafts反应。在这些反应中成功使用的亲电试剂包括各种烯酮,醛和羧酸酐。在相对较低的温度(室温或50℃)下,在乙腈中使用适度的催化剂负载量(0.025或0.1当量),反应的收率通常很高。使用的催化剂设计有联芳基支架,因此,如果确实证明它是有效的卤素键供体有机催化剂,则其对映体富集的形式可能会用作立体选择性催化剂。









































 京公网安备 11010802027423号
京公网安备 11010802027423号