当前位置:
X-MOL 学术
›
Acta Cryst. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
First COVID-19 molecular docking with a chalcone-based compound: synthesis, single-crystal structure and Hirshfeld surface analysis study
Acta Crystallographica Section C ( IF 0.7 ) Pub Date : 2020-11-05 , DOI: 10.1107/s2053229620014217 Mona A. Alsafi , David L. Hughes , Musa A. Said
Acta Crystallographica Section C ( IF 0.7 ) Pub Date : 2020-11-05 , DOI: 10.1107/s2053229620014217 Mona A. Alsafi , David L. Hughes , Musa A. Said
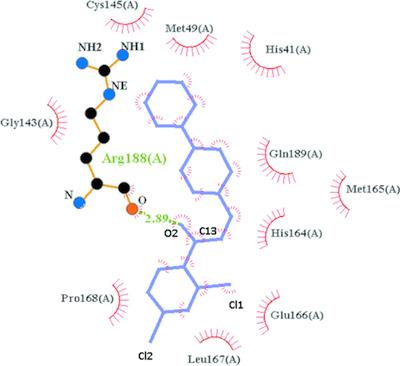
|
The first example of molecular docking of the SARS‐CoV‐2 main protease for COVID‐19 [Mpro, Protein Data Bank (PDB) code 7BQY] by a chalcone‐based ligand, namely, (E)‐1‐(2,4‐dichlorophenyl)‐3‐[4‐(morpholin‐4‐yl)phenyl]prop‐2‐en‐1‐one, C19H17Cl2NO2, I, is presented. Two‐dimensional (2D) LIGPLOT representations calculated for the inhibitor N3, viz. N‐{[(5‐methylisoxazol‐3‐yl)carbonyl]alanyl}‐l‐valyl‐N1‐((1R,2Z)‐4‐(benzyloxy)‐4‐oxo‐1‐{[(3R)‐2‐oxopyrrolidin‐3‐yl]methyl}but‐2‐enyl)‐l‐leucinamide, and 7BQY are included for comparison with our chalcone‐based complexes. The binding affinity of our chalcone ligand with 7BQY is −7.0 kcal mol−1, a high value which was attributed to the presence of a hydrogen bond, together with many hydrophobic interactions between the drug and the active amino acid residues of the receptor. Docking studies were also performed, employing rigid and flexible binding modes for the ligand. The superposition of N3 and the chalcone docked into the binding pocket of 7BQY is also presented. The synthesis, single‐crystal structure, Hirshfeld surface analysis (HSA) and spectral characterization of heterocyclic chalcone‐based compound I, are also presented. The molecules are stacked, with normal π–π interactions, in the crystal.
中文翻译:

首次将基于查尔酮的化合物进行COVID-19分子对接:合成,单晶结构和Hirshfeld表面分析研究
SARS‐CoV‐2主蛋白酶通过查尔酮基配体分子对接COVID‐19 [M pro,蛋白质数据库(PDB)代码7BQY]的分子对接的第一个示例,即(E)-1-(2,给出了4-二氯苯基)-3- [4-(吗啉-4-基)苯基] prop-2-en-1-one,C 19 H 17 Cl 2 NO 2,I。为抑制剂N3计算的二维(2D)LIGPLOT表示,即。N -{[((5-甲基异恶唑-3-基)羰基]丙氨酰} l-戊基-N 1 -(((1 R,2 Z)-4-(苄氧基)-4-氧代-1-[{(3 [R)-2-氧杂吡咯烷酮-3-基]甲基}但是-2-烯基)-1-亮氨酸酰胺和7BQY与我们基于查尔酮的配合物进行比较。我们查尔酮配体与7BQY的结合亲和力为-7.0 kcal mol -1,这是一个高值,这归因于氢键的存在以及药物与受体的活性氨基酸残基之间的许多疏水相互作用。还进行了对接研究,对配体采用了刚性和柔性结合模式。还介绍了N3和查尔酮对接在7BQY的结合口袋中的叠加现象。杂环查尔酮基化合物I的合成,单晶结构,Hirshfeld表面分析(HSA)和光谱表征,也介绍了。分子以正常的π-π相互作用堆叠在晶体中。
更新日期:2020-12-04
中文翻译:

首次将基于查尔酮的化合物进行COVID-19分子对接:合成,单晶结构和Hirshfeld表面分析研究
SARS‐CoV‐2主蛋白酶通过查尔酮基配体分子对接COVID‐19 [M pro,蛋白质数据库(PDB)代码7BQY]的分子对接的第一个示例,即(E)-1-(2,给出了4-二氯苯基)-3- [4-(吗啉-4-基)苯基] prop-2-en-1-one,C 19 H 17 Cl 2 NO 2,I。为抑制剂N3计算的二维(2D)LIGPLOT表示,即。N -{[((5-甲基异恶唑-3-基)羰基]丙氨酰} l-戊基-N 1 -(((1 R,2 Z)-4-(苄氧基)-4-氧代-1-[{(3 [R)-2-氧杂吡咯烷酮-3-基]甲基}但是-2-烯基)-1-亮氨酸酰胺和7BQY与我们基于查尔酮的配合物进行比较。我们查尔酮配体与7BQY的结合亲和力为-7.0 kcal mol -1,这是一个高值,这归因于氢键的存在以及药物与受体的活性氨基酸残基之间的许多疏水相互作用。还进行了对接研究,对配体采用了刚性和柔性结合模式。还介绍了N3和查尔酮对接在7BQY的结合口袋中的叠加现象。杂环查尔酮基化合物I的合成,单晶结构,Hirshfeld表面分析(HSA)和光谱表征,也介绍了。分子以正常的π-π相互作用堆叠在晶体中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号