当前位置:
X-MOL 学术
›
Int. J. Quantum Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical studies on how to tune the π‐hole pnicogen bonds by substitution and cooperative effects
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2020-11-04 , DOI: 10.1002/qua.26531 Lijuan Zhang 1 , Dazhi Li 1
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2020-11-04 , DOI: 10.1002/qua.26531 Lijuan Zhang 1 , Dazhi Li 1
Affiliation
|
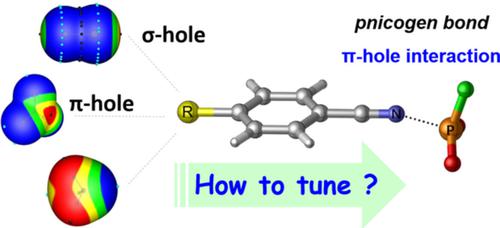
A systemic investigation of the substitution and cooperative effects on the P…N π‐hole pnicogen bond were performed via theoretical calculations. The structural and energetic properties of the binary complexes between a series of substituted benzonitrile and PO2F have been examined to study the substitution effect. The stability of the binary complexes increases in the order of CN < Br ≈ Cl < F < H < CH3 < NH2, in which all halogen atoms serve as the electron‐withdrawing substituents. The cooperativity between halogen/triel bond with pnicogen bond were also studied in X…NC‐Ph‐CN…PO2F and Y…Br‐Ph‐CN…PO2F complexes with X = F2, Cl2, Br2, FCl, FBr, BrCl, FCN, ClCN, BrCN, BH3, BF3, and Y = NH3, NH2CH3, NHCH2, HCN. It is found that the mutual effect of the interactions is weakened through the phenyl ring relative to the heterocyclic ring. The molecular electrostatic potential, atoms in molecules and natural bond orbital analysis were carried out to unveil the nature of these interactions. Several linear relationships have been established between the geometric parameters and the interaction energies or some electronic properties of the complexes. The distinct differences for the complexes involving BH3 and their BF3 analogs may be attributed to the greater π electron density on the BF3 moiety, and the underlying reasons are discussed in detail.
中文翻译:

关于如何通过置换和协同效应来调节π孔Pnicogen键的理论研究
通过理论计算,对P…Nπ孔pogenogen键的取代和协同作用进行了系统研究。研究了一系列取代的苄腈和PO 2 F之间的二元配合物的结构和能量性质,以研究取代作用。二元配合物的稳定性按CN <Br≈Cl <F <H <CH 3 <NH 2的顺序增加,其中所有卤素原子均用作吸电子取代基。还研究了X = NC 2,Cl 2,Br 2的X…NC‐Ph‐CN…PO 2 F和Y…Br‐Ph‐CN…PO 2 F配合物中卤素/三醇键与发烟剂键之间的协同作用。,FCl,FBr,BrCl,FCN,ClCN,BrCN,BH 3,BF 3,并且Y = NH 3,NH 2 CH 3,NHCH 2,HCN。已经发现,通过苯环相对于杂环减弱了相互作用的相互作用。进行了分子静电势,分子中的原子和自然键轨道分析以揭示这些相互作用的性质。在几何参数与配合物的相互作用能或某些电子性质之间建立了一些线性关系。涉及BH 3及其BF 3的配合物的明显差异类似物可以归因于BF 3部分上的更大的π电子密度,并且详细讨论了根本原因。
更新日期:2020-11-04
中文翻译:

关于如何通过置换和协同效应来调节π孔Pnicogen键的理论研究
通过理论计算,对P…Nπ孔pogenogen键的取代和协同作用进行了系统研究。研究了一系列取代的苄腈和PO 2 F之间的二元配合物的结构和能量性质,以研究取代作用。二元配合物的稳定性按CN <Br≈Cl <F <H <CH 3 <NH 2的顺序增加,其中所有卤素原子均用作吸电子取代基。还研究了X = NC 2,Cl 2,Br 2的X…NC‐Ph‐CN…PO 2 F和Y…Br‐Ph‐CN…PO 2 F配合物中卤素/三醇键与发烟剂键之间的协同作用。,FCl,FBr,BrCl,FCN,ClCN,BrCN,BH 3,BF 3,并且Y = NH 3,NH 2 CH 3,NHCH 2,HCN。已经发现,通过苯环相对于杂环减弱了相互作用的相互作用。进行了分子静电势,分子中的原子和自然键轨道分析以揭示这些相互作用的性质。在几何参数与配合物的相互作用能或某些电子性质之间建立了一些线性关系。涉及BH 3及其BF 3的配合物的明显差异类似物可以归因于BF 3部分上的更大的π电子密度,并且详细讨论了根本原因。











































 京公网安备 11010802027423号
京公网安备 11010802027423号