当前位置:
X-MOL 学术
›
ChemNanoMat
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A New Pressure‐induced Mechanism of Polyvinyl Chloride Pyrolysis with Formation of Nanodiamonds
ChemNanoMat ( IF 2.6 ) Pub Date : 2020-11-05 , DOI: 10.1002/cnma.202000504 M. V. Kondrin 1 , I. P. Zibrov 1 , S. G. Lyapin 1 , Yu. V. Grigoriev 2 , R. A. Khmelnitskiy 3 , E. A. Ekimov 1, 3
ChemNanoMat ( IF 2.6 ) Pub Date : 2020-11-05 , DOI: 10.1002/cnma.202000504 M. V. Kondrin 1 , I. P. Zibrov 1 , S. G. Lyapin 1 , Yu. V. Grigoriev 2 , R. A. Khmelnitskiy 3 , E. A. Ekimov 1, 3
Affiliation
|
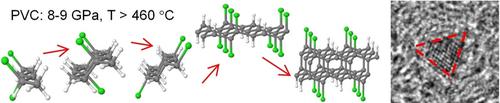
Application of high pressure to molecular compounds with high compressibility can initiate chemical reactions, unexpected under normal conditions. Here we present the evidence of pressure‐induced change in the mechanism of dehydrochlorination at polyvinyl chloride (PVC) pyrolysis. PVC pyrolysis was investigated at pressures from 2 to 9 GPa and temperatures up to 1600 °C. At critical pressures of 8–9 GPa, decomposition of PVC starts at 500 °C and results in formation of ultrasmall nanodiamonds. Based on density functional theory calculations, we show that intermolecular linking by single C−C bonds during dehydrochlorination is responsible for the scenario of carbonization, leading to nanodiamonds formation. At decreased pressure, carbonized product consists mainly of graphite‐like carbon, implying that high‐pressure intermolecular dehydrochlorination of PVC becomes inferior to “normal”, intramolecular one. Our study suggests that at low pressures, random intermolecular linking of PVC with formation of single HCl molecules may trigger “normal”, HCl‐catalyzed dehydrochlorination.
中文翻译:

纳米金刚石形成的聚氯乙烯热解压力诱导新机理
对具有高可压缩性的分子化合物施加高压会引发化学反应,这在正常情况下是无法预料的。在这里,我们提供了聚氯乙烯(PVC)热解过程中压力引起的脱氯化氢机理变化的证据。在2至9 GPa的压力和高达1600°C的温度下研究了PVC热解。在8–9 GPa的临界压力下,PVC的分解从500°C开始,并导致形成超小纳米金刚石。根据密度泛函理论计算,我们表明脱氯化氢过程中单个C-C键的分子间连接是导致碳化的场景,导致纳米金刚石的形成。在减压下,碳化产物主要由类石墨碳组成,这意味着PVC的高压分子间脱氯化氢性能不如“正常”分子内氯化氢。我们的研究表明,在低压下,PVC的分子间随机连接与单个HCl分子的形成可能会触发“正常”的,HCl催化的脱氯化氢反应。
更新日期:2021-01-12
中文翻译:

纳米金刚石形成的聚氯乙烯热解压力诱导新机理
对具有高可压缩性的分子化合物施加高压会引发化学反应,这在正常情况下是无法预料的。在这里,我们提供了聚氯乙烯(PVC)热解过程中压力引起的脱氯化氢机理变化的证据。在2至9 GPa的压力和高达1600°C的温度下研究了PVC热解。在8–9 GPa的临界压力下,PVC的分解从500°C开始,并导致形成超小纳米金刚石。根据密度泛函理论计算,我们表明脱氯化氢过程中单个C-C键的分子间连接是导致碳化的场景,导致纳米金刚石的形成。在减压下,碳化产物主要由类石墨碳组成,这意味着PVC的高压分子间脱氯化氢性能不如“正常”分子内氯化氢。我们的研究表明,在低压下,PVC的分子间随机连接与单个HCl分子的形成可能会触发“正常”的,HCl催化的脱氯化氢反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号