当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Activating Iron Based Materials for Overall Electrochemical Water Splitting via the Incorporation of Noble Metals
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2020-11-04 , DOI: 10.1002/asia.202001113 Md Abu Sayeed 1, 2 , Jonathan Heron 1 , Jonathan Love 1, 3 , Anthony P. O'Mullane 1, 2, 3
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2020-11-04 , DOI: 10.1002/asia.202001113 Md Abu Sayeed 1, 2 , Jonathan Heron 1 , Jonathan Love 1, 3 , Anthony P. O'Mullane 1, 2, 3
Affiliation
|
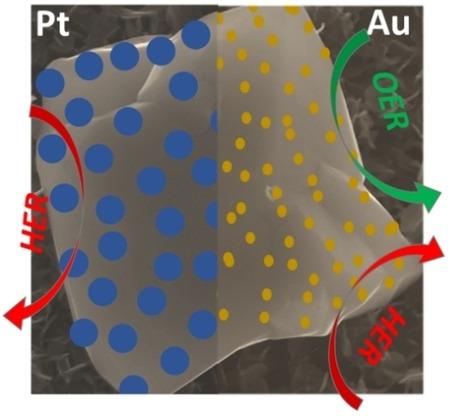
Although the presence of iron in mixed metal oxide based catalysts has shown significant performance improvement in the oxygen evolution reaction (OER), iron oxides themselves demonstrate much poorer activity. In this study, we investigate improving the performance of iron catalysts via surface decoration with gold or platinum for not only the OER but also the hydrogen evolution reaction (HER) for overall water splitting in an alkaline electrolyte. Two types of iron catalysts were synthesised, iron nanocubes and iron oxide via electrochemical deposition methods which were decorated with either Au or Pt via galvanic replacement. It was found that the presence of Au significantly enhanced the OER performance of iron oxide and the HER performance of iron nanocubes. The presence of Pt resulted in moderate improvement in the OER but significant improvement for the HER but did not surpass the performance of gold decorated iron nanocubes. This indicates that the speciation of the iron catalyst and the decorating metal was important for tuning the activity to the OER and the HER. For the OER, the formation of iron oxide/Au interfaces was determined to be an important component for high activity whereas the metallic nature of metal decorated iron nanocubes was important for the HER. Therefore, iron based catalysts can be modified to demonstrate bifunctional behaviour for overall water splitting via the inclusion of gold nanoparticles.
中文翻译:

通过引入贵金属活化铁基材料进行整体电化学水分解
尽管铁在混合金属氧化物基催化剂中的存在已显示出氧释放反应(OER)的显着性能改善,但铁氧化物本身却显示出较差的活性。在这项研究中,我们研究了通过用金或铂进行表面装饰来提高铁催化剂的性能,这些效果不仅可以用于OER,还可以用于氢分解反应(HER),用于在碱性电解质中进行整体水分解。通过电化学沉积方法合成了两种类型的铁催化剂:铁纳米立方体和氧化铁,它们通过电置换用Au或Pt装饰。发现Au的存在显着增强了氧化铁的OER性能和铁纳米立方体的HER性能。Pt的存在可导致OER适度改善,但HER的显着改善,但未超过金饰铁纳米立方体的性能。这表明铁催化剂和装饰金属的形态对于调节对OER和HER的活性很重要。对于OER,氧化铁/ Au界面的形成被确定为高活性的重要组成部分,而金属装饰的铁纳米立方体的金属性质对HER很重要。因此,可以修饰铁基催化剂以通过包含金纳米颗粒来证明整体水分解的双功能行为。这表明铁催化剂和装饰金属的形态对于调节对OER和HER的活性很重要。对于OER,氧化铁/ Au界面的形成被确定为高活性的重要组成部分,而金属装饰的铁纳米立方体的金属性质对HER很重要。因此,可以修饰铁基催化剂以通过包含金纳米颗粒来证明整体水分解的双功能行为。这表明铁催化剂和装饰金属的形态对于调节对OER和HER的活性很重要。对于OER,氧化铁/ Au界面的形成被确定为高活性的重要组成部分,而金属装饰的铁纳米立方体的金属性质对HER很重要。因此,可以修饰铁基催化剂以通过包含金纳米颗粒来证明整体水分解的双功能行为。
更新日期:2020-12-16
中文翻译:

通过引入贵金属活化铁基材料进行整体电化学水分解
尽管铁在混合金属氧化物基催化剂中的存在已显示出氧释放反应(OER)的显着性能改善,但铁氧化物本身却显示出较差的活性。在这项研究中,我们研究了通过用金或铂进行表面装饰来提高铁催化剂的性能,这些效果不仅可以用于OER,还可以用于氢分解反应(HER),用于在碱性电解质中进行整体水分解。通过电化学沉积方法合成了两种类型的铁催化剂:铁纳米立方体和氧化铁,它们通过电置换用Au或Pt装饰。发现Au的存在显着增强了氧化铁的OER性能和铁纳米立方体的HER性能。Pt的存在可导致OER适度改善,但HER的显着改善,但未超过金饰铁纳米立方体的性能。这表明铁催化剂和装饰金属的形态对于调节对OER和HER的活性很重要。对于OER,氧化铁/ Au界面的形成被确定为高活性的重要组成部分,而金属装饰的铁纳米立方体的金属性质对HER很重要。因此,可以修饰铁基催化剂以通过包含金纳米颗粒来证明整体水分解的双功能行为。这表明铁催化剂和装饰金属的形态对于调节对OER和HER的活性很重要。对于OER,氧化铁/ Au界面的形成被确定为高活性的重要组成部分,而金属装饰的铁纳米立方体的金属性质对HER很重要。因此,可以修饰铁基催化剂以通过包含金纳米颗粒来证明整体水分解的双功能行为。这表明铁催化剂和装饰金属的形态对于调节对OER和HER的活性很重要。对于OER,氧化铁/ Au界面的形成被确定为高活性的重要组成部分,而金属装饰的铁纳米立方体的金属性质对HER很重要。因此,可以修饰铁基催化剂以通过包含金纳米颗粒来证明整体水分解的双功能行为。











































 京公网安备 11010802027423号
京公网安备 11010802027423号