Journal of Rare Earths ( IF 5.2 ) Pub Date : 2020-11-05 , DOI: 10.1016/j.jre.2020.10.021 Wenjuan Cao , Kun Huang , Xiaoqin Wang , Huizhou Liu
|
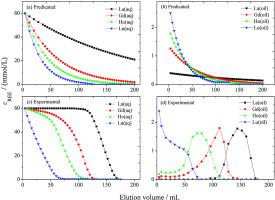
According to the tetrad-effect, 14 elements of lanthanides can be divided into four groups. In our previous study, a new approach was proposed for the kinetic separation of four rare earth ions La(III), Gd(III), Ho(III) and Lu(III) coming from four groups. In that study, four rare-earth ions were kinetically separated from their coexisting mixed aqueous solutions, by performing liquid-column elution using the aqueous solution containing four lanthanide rare-earth ions as the stationary phase and the dispersed organic oil droplets containing HEHEHP (2-ethyl hexyl phosphonic acid mono 2-ethyl hexyl ester) extractant as the mobile phase. The study of extraction kinetics is very important for understanding the kinetic separation of rare earth ions, which was carried out in this paper. The extraction kinetics of La(III), Gd(III), Ho(III) and Lu(III) by HEHEHP diluted in heptane were investigated using single drop method. The different parameters affecting the extraction rate such as column length, specific interfacial area, rare earth ion concentration, extractant concentration, hydrogen ion concentration and temperature were separately studied and the rate equations are deduced. It is first order with respect to rare earth ion and HEHEHP concentrations, and negative first order with respect to hydrogen ion concentrations. The rate constants at 293.15 K are 10−6.23, 10−5.73, 10−5.58 and 10−5.43, respectively. The experimental results demonstrate that the extraction rate of La(III), Gd(III), Ho(III) or Lu(III) is diffusion-controlled, and the extraction reaction takes place at the interface rather than in the bulk phase. The extraction model was proposed. Besides, the kinetic separation of rare earth ions by HEHEHP oil drops was discussed.
中文翻译:

HEHEHP 从氯化物介质中提取 La(III)、Gd(III)、Ho(III) 和 Lu(III) 的动力学和动力学分离
根据四分效应,镧系元素的14种元素可分为四组。在我们之前的研究中,提出了一种新方法来动态分离来自四组的四种稀土离子 La(III)、Gd(III)、Ho(III) 和 Lu(III)。在该研究中,通过使用含有四种镧系元素稀土离子的水溶液作为固定相和含有 HEHEHP (2 -乙基己基膦酸单2-乙基己酯)萃取剂作为流动相。萃取动力学的研究对于理解稀土离子的动力学分离非常重要,本文开展了这一研究。La(III)、Gd(III)、使用单滴法研究在庚烷中稀释的 HEHEHP 的 Ho(III) 和 Lu(III)。分别研究了柱长、比界面面积、稀土离子浓度、萃取剂浓度、氢离子浓度和温度等影响萃取率的不同参数,推导了萃取率方程。稀土离子和 HEHEHP 浓度为一阶,氢离子浓度为负一阶。293.15 K 时的速率常数为 10 分别研究了氢离子浓度和温度,推导了速率方程。稀土离子和 HEHEHP 浓度为一阶,氢离子浓度为负一阶。293.15 K 时的速率常数为 10 分别研究了氢离子浓度和温度,推导了速率方程。稀土离子和 HEHEHP 浓度为一阶,氢离子浓度为负一阶。293.15 K 时的速率常数为 10-6.23、10 -5.73、10 -5.58和10 -5.43分别。实验结果表明,La(III)、Gd(III)、Ho(III) 或 Lu(III) 的萃取速率受扩散控制,萃取反应发生在界面而不是体相。提出了提取模型。此外,还讨论了HEHEHP油滴对稀土离子的动力学分离。











































 京公网安备 11010802027423号
京公网安备 11010802027423号