Chemical Physics Letters ( IF 2.8 ) Pub Date : 2020-11-04 , DOI: 10.1016/j.cplett.2020.138140 Ryuichi Wada , Kenichi Tonokura , Shohei Koba , Tomonobu Imamura , Kosuke Nakai , Hiroshi Ushiyama , Koichi Yamashita , Yutaka Matsumi , Shinichi Enami , Paul W. Seakins
|
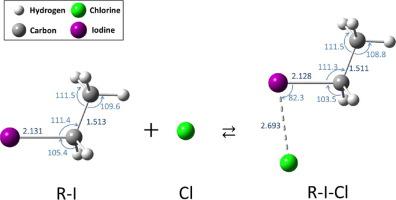
The equilibrium geometries for the ground state of the adducts formed between alkyl iodides (RI) and chlorine (Cl) atoms were optimized, and the enthalpies of adduct formations (EAFs) were obtained. The EAFs decreased when the number of alkyl substituents on the C atom bonded to I atom was increased. This trend suggests that the amount of charge transferred from the electron-donating alkyl group to I Cl increases in accordance with the number of substituents, which results in the stabilization of the adduct. Charge density distribution between the Cl and H atoms was an additional mechanism to stabilize the adducts.
Cl increases in accordance with the number of substituents, which results in the stabilization of the adduct. Charge density distribution between the Cl and H atoms was an additional mechanism to stabilize the adducts.
中文翻译:

烷基碘与氯原子之间加合物形成焓的理论研究
优化了烷基碘(RI)和氯(Cl)原子之间形成的加合物基态的平衡几何构型,并获得了加合物形成的焓(EAF)。当与I原子键合的C原子上的烷基取代基数量增加时,EAF降低。这种趋势表明,从供电子烷基转移到ICl的电荷量 根据取代基的数量而增加,这导致加合物的稳定化。Cl和H原子之间的电荷密度分布是稳定加合物的另一种机制。
根据取代基的数量而增加,这导致加合物的稳定化。Cl和H原子之间的电荷密度分布是稳定加合物的另一种机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号