Catalysis Communications ( IF 3.4 ) Pub Date : 2020-11-05 , DOI: 10.1016/j.catcom.2020.106218 N.I. Kuznetsova , D.E. Babushkin , V.N. Zudin , O.S. Koscheeva , L.I. Kuznetsova
|
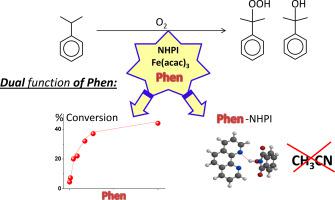
Highly efficient oxidation of isopropylbenzene mediated by the system of NHPI/Fe(acac)3/Phen has been carried out at temperature as low as 60 °C. Significant improvement of catalysis by NHPI was associated with an enhanced oxidizing ability of Fe(III) tandem with Phen, which caused the intense generation of PINO. Furthermore, NMR observations revealed formation of a hydrogen-bonded NHPI-Phen adduct soluble in acetonitrile and isopropylbenzene. Based on this phenomenon, the system was applicable for the oxidation of solvent-free isopropylbenzene. The promise of the system of NHPI/Fe(acac)3/Phen for the selective synthesis of isopropylbenzene hydroperoxide was demonstrated by oxidation at a low content of Fe(acac)3.
中文翻译:

NHPI,Fe(acac)3和1,10-菲咯啉体系介导的异丙基苯的低温氧化
由NHPI / Fe(acac)3 / Phen系统介导的异丙基苯的高效氧化已在低至60°C的温度下进行。NHPI催化的显着改善与Fe(III)与Phen串联的氧化能力增强有关,这引起了PINO的强烈生成。此外,NMR观察表明可溶于乙腈和异丙苯的氢键结合的NHPI-Phen加合物形成。基于此现象,该系统适用于无溶剂异丙苯的氧化。NHPI / Fe(acac)3 / Phen体系选择性合成氢过氧化物异丙苯的前景已通过在低含量的Fe(acac)3下进行氧化得到了证明。











































 京公网安备 11010802027423号
京公网安备 11010802027423号