Cancer Cell ( IF 48.8 ) Pub Date : 2020-11-05 , DOI: 10.1016/j.ccell.2020.10.011 Robert J Motzer 1 , Romain Banchereau 2 , Habib Hamidi 2 , Thomas Powles 3 , David McDermott 4 , Michael B Atkins 5 , Bernard Escudier 6 , Li-Fen Liu 2 , Ning Leng 2 , Alexander R Abbas 2 , Jinzhen Fan 2 , Hartmut Koeppen 2 , Jennifer Lin 2 , Susheela Carroll 7 , Kenji Hashimoto 8 , Sanjeev Mariathasan 2 , Marjorie Green 2 , Darren Tayama 2 , Priti S Hegde 9 , Christina Schiff 2 , Mahrukh A Huseni 2 , Brian Rini 10
|
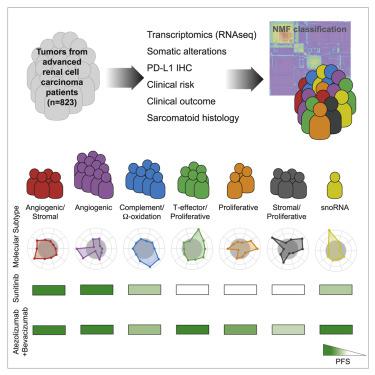
Integrated multi-omics evaluation of 823 tumors from advanced renal cell carcinoma (RCC) patients identifies molecular subsets associated with differential clinical outcomes to angiogenesis blockade alone or with a checkpoint inhibitor. Unsupervised transcriptomic analysis reveals seven molecular subsets with distinct angiogenesis, immune, cell-cycle, metabolism, and stromal programs. While sunitinib and atezolizumab + bevacizumab are effective in subsets with high angiogenesis, atezolizumab + bevacizumab improves clinical benefit in tumors with high T-effector and/or cell-cycle transcription. Somatic mutations in PBRM1 and KDM5C associate with high angiogenesis and AMPK/fatty acid oxidation gene expression, while CDKN2A/B and TP53 alterations associate with increased cell-cycle and anabolic metabolism. Sarcomatoid tumors exhibit lower prevalence of PBRM1 mutations and angiogenesis markers, frequent CDKN2A/B alterations, and increased PD-L1 expression. These findings can be applied to molecularly stratify patients, explain improved outcomes of sarcomatoid tumors to checkpoint blockade versus antiangiogenics alone, and develop personalized therapies in RCC and other indications.
中文翻译:

肾癌中的分子亚群决定检查点和血管生成阻断的结果
对来自晚期肾细胞癌 (RCC) 患者的 823 种肿瘤的综合多组学评估确定了与单独阻断血管生成或使用检查点抑制剂的不同临床结果相关的分子亚群。无监督转录组学分析揭示了七个具有不同血管生成、免疫、细胞周期、代谢和基质程序的分子亚群。虽然舒尼替尼和阿特珠单抗 + 贝伐珠单抗对高血管生成的亚群有效,但阿特珠单抗 + 贝伐珠单抗可改善具有高 T 效应子和/或细胞周期转录的肿瘤的临床益处。PBRM1和KDM5C 的体细胞突变与高血管生成和 AMPK/脂肪酸氧化基因表达相关,而CDKN2A/B和TP53改变与增加的细胞周期和合成代谢有关。肉瘤样肿瘤表现出较低的PBRM1突变和血管生成标志物发生率、频繁的CDKN2A / B改变以及增加的 PD-L1 表达。这些发现可用于对患者进行分子分层,解释肉瘤样肿瘤与单独使用抗血管生成药物相比检查点阻断治疗的改善结果,并在 RCC 和其他适应症中开发个性化治疗。











































 京公网安备 11010802027423号
京公网安备 11010802027423号