Protein & Peptide Letters ( IF 1.0 ) Pub Date : 2020-09-30 , DOI: 10.2174/0929866527666200403082721 Dinesh Chand Agrawal 1 , Anjali Yadav 1 , Mohd. Asim Khan 2 , Suman Kundu 2 , Arvind M. Kayastha 1
|
Background: β-Amylase (EC 3.2.1.2) is a maltogenic enzyme, which releases β-maltose from the non-reducing end of the substrates. The enzyme plays important roles for the production of vaccine, maltiol and maltose rich syrups. Apart from these applications the enzyme protects cells from abiotic as well as oxidative damage. The enzyme is βwell characterized in βplants and microbes and crystal structures of β-amylases βhave been βobtained from sweet potato, soybean and Bacillus cereus.
Objective: Find out correlation between structural and functional stability induced by change in pH, temperature and chaotropes.
Methods: Activity, intrinsic fluorescence, extrinsic fluorescence, near- and far- ultraviolet circular dichroism spectroscopic measurements were performed.
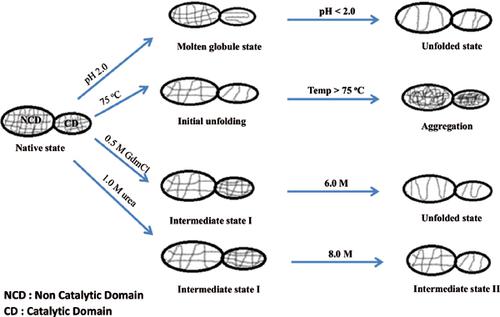
Results: Peaks about 208 nm and 222 nm obtained by near-ultraviolet circular dichroism correspond to α-helix whereas peak at 215 nm shows presence of β-sheet. At pH 2.0, absence of tertiary structures, exposed of hydrophobic regions and presence of substantial secondary structures, revealed the existence of molten globule like state. Temperature induced denaturation studies showed that the enzyme was stable up to 75 ºC and the process was found to be irreversible in nature. Chaotropes dependent equilibrium unfolding studies revealed that at low concentration of chaotropes, ellipticity and intrinsic fluorescence βintensity were βdecreased βwhereas βenzymatic activity remained unchanged, which revealed fenugreek β-amylase is multi-domains enzyme and catalytic βdomain βis more βstable compare to non-catalytic domain. Moreover, the transition was sigmoidal and non-coincidental.
Conclusion: Results indicate the probable existence of intermediate states that might perform significant role in physiological process and biotechnological applications.
中文翻译:

低pH下发芽的胡芦巴β-淀粉酶揭示的熔融小球的变性诱导的平衡展开和构象过渡研究
背景:β-淀粉酶(EC 3.2.1.2)是一种产麦芽酶,可从底物的非还原末端释放β-麦芽糖。该酶在生产疫苗,麦芽酚和富含麦芽糖的糖浆中起重要作用。除这些应用外,该酶还保护细胞免受非生物以及氧化损伤。该酶在β植物和微生物中具有良好的β特性,并且β-淀粉酶的晶体结构是从甘薯,大豆和蜡状芽孢杆菌中获得的。
目的:找出pH,温度和离液剂的变化引起的结构和功能稳定性之间的相关性。
方法:进行活性,内在荧光,外在荧光,近紫外和远紫外圆二色性光谱测量。
结果:通过近紫外圆二色性获得的约208nm和222nm的峰对应于α-螺旋,而在215nm的峰表明存在β-折叠。在pH 2.0下,不存在三级结构,不暴露疏水区域,并且存在大量二级结构,这表明存在熔融的球状状态。温度诱导的变性研究表明,该酶在最高75ºC的温度下稳定,并且发现该过程本质上是不可逆的。依赖于离液剂的平衡展开研究表明,在低离液剂浓度下,椭圆率和固有荧光β强度降低了β强度,而β酶活性保持不变,这表明胡芦巴β-淀粉酶是多结构域酶,而催化β结构域β与非催化结构域相比更稳定。此外,
结论:结果表明可能存在中间状态,这些中间状态可能在生理过程和生物技术应用中起重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号