当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of Ionic Strength on Hydrophobic Interactions in Water: Dependence on Solute Size and Shape
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-11-04 , DOI: 10.1021/acs.jpcb.0c06399 Małgorzata Bogunia 1 , Mariusz Makowski 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-11-04 , DOI: 10.1021/acs.jpcb.0c06399 Małgorzata Bogunia 1 , Mariusz Makowski 1
Affiliation
|
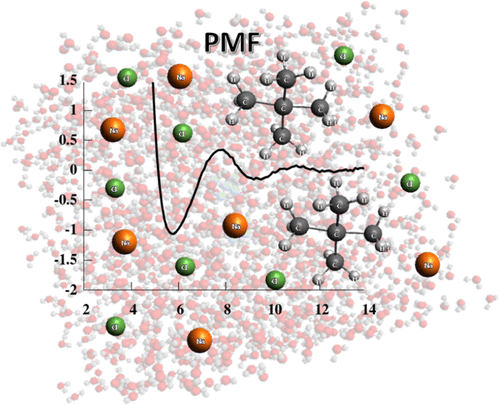
Hydrophobicity is a phenomenon of great importance in biology, chemistry, and biochemistry. It is defined as the interaction between nonpolar molecules or groups in water and their low solubility. Hydrophobic interactions affect many processes in water, for example, complexation, surfactant aggregation, and coagulation. These interactions play a pivotal role in the formation and stability of proteins or biological membranes. In the present study, we assessed the effect of ionic strength, solute size, and shape on hydrophobic interactions between pairs of nonpolar particles. Pairs of methane, neopentane, adamantane, fullerene, ethane, propane, butane, hexane, octane, and decane were simulated by molecular dynamics in AMBER 16.0 force field. As a solvent, TIP3P and TIP4PEW water models were used. Potential of mean force (PMF) plots of these dimers were determined at four values of ionic strength, 0, 0.04, 0.08, and 0.40 mol/dm3, to observe its impact on hydrophobic interactions. The characteristic shape of PMFs with three extrema (contact minimum, solvent-separated minimum, and desolvation maximum) was observed for most of the compounds for hydrophobic interactions. Ionic strength affected hydrophobic interactions. We observed a tendency to deepen contact minima with an increase in ionic strength value in the case of spherical and spheroidal molecules. Additionally, two-dimensional distribution functions describing water density and average number of hydrogen bonds between water molecules were calculated in both water models for adamantane and hexane. It was observed that the density of water did not significantly change with the increase in ionic strength, but the average number of hydrogen bonds changed. The latter tendency strongly depends on the water model used for simulations.
中文翻译:

离子强度对水中疏水相互作用的影响:取决于溶质的大小和形状
疏水性是在生物学,化学和生物化学中非常重要的现象。它定义为水中非极性分子或基团与低溶解度之间的相互作用。疏水相互作用影响水中的许多过程,例如络合,表面活性剂聚集和凝结。这些相互作用在蛋白质或生物膜的形成和稳定性中起关键作用。在本研究中,我们评估了离子强度,溶质大小和形状对非极性粒子对之间疏水相互作用的影响。在AMBER 16.0力场中通过分子动力学模拟了甲烷,新戊烷,金刚烷,富勒烯,乙烷,丙烷,丁烷,己烷,辛烷和癸烷对。作为溶剂,使用了TIP3P和TIP4PEW水模型。3,以观察其对疏水相互作用的影响。对于大多数疏水相互作用的化合物,观察到具有三个极值(接触最小,溶剂分离的最小和去溶剂化的最大)的PMF的特征形状。离子强度影响疏水相互作用。我们观察到在球形和球形分子的情况下,随着离子强度值的增加,接触最小值趋于加深的趋势。另外,在金刚烷和己烷的两种水模型中都计算了描述水密度和水分子之间氢键平均数的二维分布函数。观察到水的密度没有随着离子强度的增加而显着变化,但是氢键的平均数目发生了变化。
更新日期:2020-11-19
中文翻译:

离子强度对水中疏水相互作用的影响:取决于溶质的大小和形状
疏水性是在生物学,化学和生物化学中非常重要的现象。它定义为水中非极性分子或基团与低溶解度之间的相互作用。疏水相互作用影响水中的许多过程,例如络合,表面活性剂聚集和凝结。这些相互作用在蛋白质或生物膜的形成和稳定性中起关键作用。在本研究中,我们评估了离子强度,溶质大小和形状对非极性粒子对之间疏水相互作用的影响。在AMBER 16.0力场中通过分子动力学模拟了甲烷,新戊烷,金刚烷,富勒烯,乙烷,丙烷,丁烷,己烷,辛烷和癸烷对。作为溶剂,使用了TIP3P和TIP4PEW水模型。3,以观察其对疏水相互作用的影响。对于大多数疏水相互作用的化合物,观察到具有三个极值(接触最小,溶剂分离的最小和去溶剂化的最大)的PMF的特征形状。离子强度影响疏水相互作用。我们观察到在球形和球形分子的情况下,随着离子强度值的增加,接触最小值趋于加深的趋势。另外,在金刚烷和己烷的两种水模型中都计算了描述水密度和水分子之间氢键平均数的二维分布函数。观察到水的密度没有随着离子强度的增加而显着变化,但是氢键的平均数目发生了变化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号