当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inorganic Sulfur Species Formed upon Heterogeneous OH Oxidation of Organosulfates: A Case Study of Methyl Sulfate
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-11-04 , DOI: 10.1021/acsearthspacechem.0c00209 Rongshuang Xu 1 , Yao Ge 2 , Kai Chung Kwong 1 , Hon Yin Poon 1 , Kevin R. Wilson 3 , Jian Zhen Yu 2 , Man Nin Chan 1, 4
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-11-04 , DOI: 10.1021/acsearthspacechem.0c00209 Rongshuang Xu 1 , Yao Ge 2 , Kai Chung Kwong 1 , Hon Yin Poon 1 , Kevin R. Wilson 3 , Jian Zhen Yu 2 , Man Nin Chan 1, 4
Affiliation
|
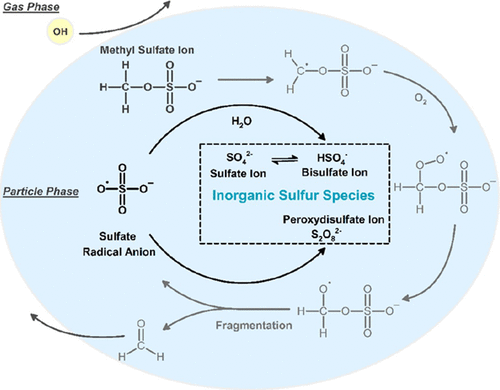
Recent studies reveal that organosulfates at the particle surface can be oxidized by gas-phase OH radicals with significant rates. Inorganic sulfur species, such as the bisulfate ion (HSO4–) and sulfate ion (SO42–), can be formed upon these heterogeneous oxidation processes through the formation and subsequent reactions of sulfate radical anions (SO4•–) in the particle phase. However, the amount of inorganic sulfur species produced in these heterogeneous oxidation reactions is not known. We investigate the heterogeneous OH oxidation of sodium methyl sulfate (CH3SO4Na), the smallest organosulfate detected in atmospheric particles, using an oxidation flow reactor at a relative humidity of 75%. We quantify the kinetics by measuring the decay of CH3SO4Na and the amount of HSO4– and SO42– formed upon oxidation using ion chromatography. Kinetic measurements determine the heterogeneous OH reaction rate to be (5.72 ± 0.14) × 10–13 cm3 molecule–1 s–1, with an effective OH uptake coefficient, γeff, of 0.31 ± 0.06. The molar yield of inorganic sulfur species, defined as the total number of moles of HSO4– and SO42– formed per mole of CH3SO4Na consumed upon oxidation, is found to be significant and has an average value of 0.62 ± 0.18 upon oxidation. A kinetic model is developed to describe the kinetics and inorganic sulfur species formation upon oxidation. Model simulations suggest that CH3SO4Na tends to decompose rapidly into formaldehyde and SO4•–, and the reaction of SO4•– with CH3SO4Na plays a significant role in both governing the kinetics and the formation of inorganic sulfur species.
中文翻译:

有机硫酸盐异质OH氧化形成的无机硫物种:硫酸甲酯的案例研究
最近的研究表明,颗粒表面的有机硫酸盐可以被气相OH自由基以高速率氧化。无机硫物质,如硫酸氢根离子(HSO 4 - ),硫酸离子(SO 4 2- ),可以在通过形成这些非均相氧化过程和硫酸根阴离子的后续反应形成(SO 4 • - )在粒子相。然而,在这些非均相氧化反应中产生的无机硫种类的数量是未知的。我们研究了甲基磺酸钠(CH 3 SO 4Na),这是在相对湿度为75%的条件下使用氧化流反应器在大气颗粒中检测到的最小有机硫酸盐。我们通过测量CH的衰减量化动力学3 SO 4 Na和HSO的量4 -和SO 4 2-离子色谱法在氧化时形成的。动力学测量确定异质OH反应速率为(5.72±0.14)×10 –13 cm 3分子–1 s –1,有效OH吸收系数γeff为0.31±0.06。无机硫物质的摩尔产率,定义为HSO 4 –和SO的总摩尔数发现每摩尔在氧化过程中消耗的CH 3 SO 4 Na形成4 2–十分重要,氧化时的平均值为0.62±0.18。建立动力学模型以描述动力学和氧化时无机硫物种的形成。模型模拟表明,CH 3 SO 4 Na倾向于迅速分解为甲醛和SO 4 •–,并且SO 4 •–与CH 3 SO 4 Na的反应在控制动力学和无机硫的形成中均起着重要作用。种类。
更新日期:2020-11-19
中文翻译:

有机硫酸盐异质OH氧化形成的无机硫物种:硫酸甲酯的案例研究
最近的研究表明,颗粒表面的有机硫酸盐可以被气相OH自由基以高速率氧化。无机硫物质,如硫酸氢根离子(HSO 4 - ),硫酸离子(SO 4 2- ),可以在通过形成这些非均相氧化过程和硫酸根阴离子的后续反应形成(SO 4 • - )在粒子相。然而,在这些非均相氧化反应中产生的无机硫种类的数量是未知的。我们研究了甲基磺酸钠(CH 3 SO 4Na),这是在相对湿度为75%的条件下使用氧化流反应器在大气颗粒中检测到的最小有机硫酸盐。我们通过测量CH的衰减量化动力学3 SO 4 Na和HSO的量4 -和SO 4 2-离子色谱法在氧化时形成的。动力学测量确定异质OH反应速率为(5.72±0.14)×10 –13 cm 3分子–1 s –1,有效OH吸收系数γeff为0.31±0.06。无机硫物质的摩尔产率,定义为HSO 4 –和SO的总摩尔数发现每摩尔在氧化过程中消耗的CH 3 SO 4 Na形成4 2–十分重要,氧化时的平均值为0.62±0.18。建立动力学模型以描述动力学和氧化时无机硫物种的形成。模型模拟表明,CH 3 SO 4 Na倾向于迅速分解为甲醛和SO 4 •–,并且SO 4 •–与CH 3 SO 4 Na的反应在控制动力学和无机硫的形成中均起着重要作用。种类。









































 京公网安备 11010802027423号
京公网安备 11010802027423号