当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stabilization of Protein–Protein Interactions between CaMKK2 and 14–3–3 by Fusicoccins
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-11-04 , DOI: 10.1021/acschembio.0c00821 Domenico Lentini Santo 1 , Olivia Petrvalska 1, 2 , Veronika Obsilova 2 , Christian Ottmann 3 , Tomas Obsil 1, 2
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-11-04 , DOI: 10.1021/acschembio.0c00821 Domenico Lentini Santo 1 , Olivia Petrvalska 1, 2 , Veronika Obsilova 2 , Christian Ottmann 3 , Tomas Obsil 1, 2
Affiliation
|
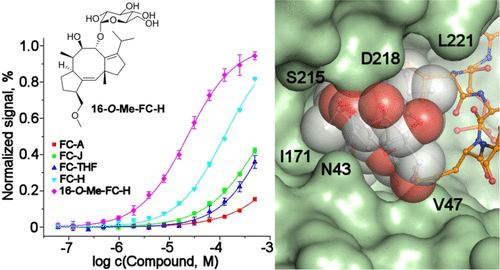
Ca2+/calmodulin-dependent protein kinase kinase 2 (CaMKK2) regulates several key physiological and pathophysiological processes, and its dysregulation has been implicated in obesity, diabetes, and cancer. CaMKK2 is inhibited through phosphorylation in a process involving binding to the scaffolding 14–3–3 protein, which maintains CaMKK2 in the phosphorylation-mediated inhibited state. The previously reported structure of the N-terminal CaMKK2 14–3–3-binding motif bound to 14–3–3 suggested that the interaction between 14–3–3 and CaMKK2 could be stabilized by small-molecule compounds. Thus, we investigated the stabilization of interactions between CaMKK2 and 14–3–3γ by Fusicoccin A and other fusicoccanes—diterpene glycosides that bind at the interface between the 14–3–3 ligand binding groove and the 14–3–3 binding motif of the client protein. Our data reveal that two of five tested fusicoccanes considerably increase the binding of phosphopeptide representing the 14–3–3 binding motif of CaMKK2 to 14–3–3γ. Crystal structures of two ternary complexes suggest that the steric contacts between the C-terminal part of the CaMKK2 14–3–3 binding motif and the adjacent fusicoccane molecule are responsible for differences in stabilization potency between the study compounds. Moreover, our data also show that fusicoccanes enhance the binding affinity of phosphorylated full-length CaMKK2 to 14–3–3γ, which in turn slows down CaMKK2 dephosphorylation, thus keeping this protein in its phosphorylation-mediated inhibited state. Therefore, targeting the fusicoccin binding cavity of 14–3–3 by small-molecule compounds may offer an alternative strategy to suppress CaMKK2 activity by stabilizing its phosphorylation-mediated inhibited state.
中文翻译:

Fusicoccins稳定CaMKK2和14-3-3之间的蛋白质-蛋白质相互作用
钙2+/钙调蛋白依赖性蛋白激酶2(CaMKK2)调节几个关键的生理和病理生理过程,其失调与肥胖,糖尿病和癌症有关。CaMKK2在涉及与支架14–3–3蛋白结合的过程中通过磷酸化被抑制,该蛋白将CaMKK2保持在磷酸化介导的抑制状态。先前报道的N末端CaMKK2 14–3–3结合基序与14–3–3结合的结构表明,小分子化合物可以稳定14–3–3和CaMKK2之间的相互作用。因此,我们研究了Fusicoccin A和其他Fusicoccanes(在萜烯的14–3–3–3配体结合凹槽和14–3–3结合基序之间的界面结合的二萜糖苷)对CaMKK2和14–3–3γ之间相互作用的稳定作用。客户蛋白质。我们的数据显示,五种经测试的呋喃二烷中有两种会大大增加代表CaMKK2的14–3–3结合基序的磷酸肽对14–3–3γ的结合。两个三元复合物的晶体结构表明,CaMKK2 14–3–3结合基序的C末端部分与相邻的富康烷分子之间的空间接触是造成研究化合物之间稳定能力差异的原因。此外,我们的数据还显示,呋喃二烷增强了磷酸化的全长CaMKK2与14–3–3γ的结合亲和力,从而减慢了CaMKK2的去磷酸化,从而使该蛋白保持在其磷酸化介导的抑制状态。因此,
更新日期:2020-11-21
中文翻译:

Fusicoccins稳定CaMKK2和14-3-3之间的蛋白质-蛋白质相互作用
钙2+/钙调蛋白依赖性蛋白激酶2(CaMKK2)调节几个关键的生理和病理生理过程,其失调与肥胖,糖尿病和癌症有关。CaMKK2在涉及与支架14–3–3蛋白结合的过程中通过磷酸化被抑制,该蛋白将CaMKK2保持在磷酸化介导的抑制状态。先前报道的N末端CaMKK2 14–3–3结合基序与14–3–3结合的结构表明,小分子化合物可以稳定14–3–3和CaMKK2之间的相互作用。因此,我们研究了Fusicoccin A和其他Fusicoccanes(在萜烯的14–3–3–3配体结合凹槽和14–3–3结合基序之间的界面结合的二萜糖苷)对CaMKK2和14–3–3γ之间相互作用的稳定作用。客户蛋白质。我们的数据显示,五种经测试的呋喃二烷中有两种会大大增加代表CaMKK2的14–3–3结合基序的磷酸肽对14–3–3γ的结合。两个三元复合物的晶体结构表明,CaMKK2 14–3–3结合基序的C末端部分与相邻的富康烷分子之间的空间接触是造成研究化合物之间稳定能力差异的原因。此外,我们的数据还显示,呋喃二烷增强了磷酸化的全长CaMKK2与14–3–3γ的结合亲和力,从而减慢了CaMKK2的去磷酸化,从而使该蛋白保持在其磷酸化介导的抑制状态。因此,










































 京公网安备 11010802027423号
京公网安备 11010802027423号