当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
β-(Z) Selectivity Control by Cyclometalated Rhodium(III)–Triazolylidene Homogeneous and Heterogeneous Terminal Alkyne Hydrosilylation Catalysts
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-11-03 , DOI: 10.1021/acscatal.0c03295 Beatriz Sánchez-Page 1 , Julen Munarriz 2, 3 , M. Victoria Jiménez 1 , Jesús J. Pérez-Torrente 1 , Javier Blasco 4, 5 , Gloria Subias 4, 5 , Vincenzo Passarelli 6 , Patricia Álvarez 7
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-11-03 , DOI: 10.1021/acscatal.0c03295 Beatriz Sánchez-Page 1 , Julen Munarriz 2, 3 , M. Victoria Jiménez 1 , Jesús J. Pérez-Torrente 1 , Javier Blasco 4, 5 , Gloria Subias 4, 5 , Vincenzo Passarelli 6 , Patricia Álvarez 7
Affiliation
|
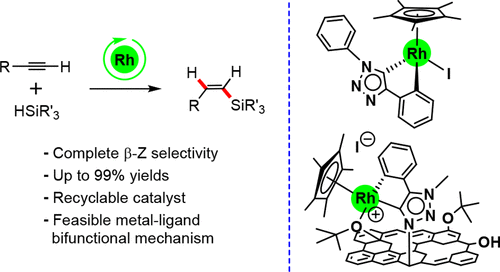
The cyclometalated Rh(III)–NHC compounds [Cp*RhI(C,C′)-Triaz] (Triaz = 1,4-diphenyl-3-methyl-1,2,3-triazol-5-ylidene) and [Cp*RhI(C,C′)-Im] (Im = 1-phenyl-3-methyl-imidazol-2-ylidene) are efficient catalysts for the hydrosilylation of terminal alkynes with complete regio- and stereoselectivity toward the thermodynamically less stable β-(Z)-vinylsilane isomer at room temperature in chloroform or acetone. Catalyst [Cp*RhI(C,C′)-Triaz] shows a superior catalytic performance in terms of activity and has been applied to the hydrosilylation of a range of linear 1-alkynes and phenylacetylene derivatives with diverse hydrosilanes, including HSiMePh2, HSiMe2Ph, HSiEt3, and the bulkier heptamethylhydrotrisiloxane (HMTS), to afford the corresponding β-(Z)-vinylsilanes in quantitative yields. The graphene-based hybrid material TRGO-Triaz-Rh(III), featuring cyclometalated [Cp*RhI(C,C′)-Triaz] (Triaz = 1,4-diphenyl-3-methyl-1,2,3-triazol-5-ylidene) rhodium(III) complexes covalently immobilized through the triazolylidene linker, has been prepared by metalation of the trimethylsilyl-protected 3-methyl-4-phenyl-1,2,3-triazolium iodide functionalized graphene oxide material, TRGO-Triaz, with [Cp*RhCl2]2 using sodium tert-butoxide as base. The coordination sphere of the supported rhodium(III) complexes has been determined by means of XPS and extended X-ray absorption fine structure (EXAFS) spectroscopy, showing the replacement of the iodido ligand by O-functionalities on the carbon wall. In sharp contrast with the homogeneous catalyst, the heterogeneous hybrid catalyst TRGO-Triaz-Rh(III) is not active at room temperature although it shows an excellent catalytic performance at 60 °C. In addition, the hybrid catalyst TRGO-Triaz-Rh(III) has shown an excellent recyclability, allowing at least six catalytic runs in the hydrosilylation of oct-1-yne with HSiMePh2 in acetone with complete selectivity to the β-(Z)-vinylsilane product. The reaction mechanism for the molecular catalyst [Cp*RhI(C,C′)-Triaz] has been explored by means of DFT calculations, pointing to a metal–ligand bifunctional mechanism involving reversible cyclometalation that is competitive with a noncooperative pathway. The proposed mechanism entails the Rh–CAr assisted hydrosilane activation to afford a reactive Rh–silyl intermediate that leads to a (E)-silylvinylene intermediate after alkyne insertion and a metallacyclopropene-driven isomerization. The release of the β-(Z)-vinylsilane product can occur by a reversible cyclometalation mechanism involving σ-CAM with the CAr–H bond or, alternatively, the Si–H bond of an external hydrosilane. The energy barrier for the latter is 1.2 kcal·mol–1 lower than that of the CAr–H bond, which results in a small energy span difference that makes both pathways competitive under catalytic conditions.
中文翻译:

环金属化铑(III)-三唑基均相和均相末端炔烃加氢硅烷化催化剂控制β-(Z)选择性
环金属化的Rh(III)-NHC化合物[Cp * RhI(C,C')-Triaz](Triaz = 1,4-二苯基-3-甲基-1,2,3-三唑-5-亚萘基)和[Cp * RhI(C,C')-Im](Im = 1-苯基-3-甲基-咪唑-2-亚烷基)是有效的催化剂,可用于末端炔烃的氢化硅烷化,对热力学不稳定的β-具有完全的区域和立体选择性(Z)-乙烯基硅烷异构体在室温下在氯仿或丙酮中。催化剂[Cp * RhI(C,C')-Triaz]在活性方面表现出优异的催化性能,已被应用于各种线性1-炔烃和苯基乙炔衍生物与各种氢化硅烷(包括HSiMePh 2,HSiMe)的氢化硅烷化反应2相,HSiEt 3,以及体积较大的七甲基氢三硅氧烷(HMTS),以定量收率得到相应的β-(Z)-乙烯基硅烷。石墨烯基杂化材料TRGO-Triaz-Rh(III),具有环金属化的[Cp * RhI(C,C')-Triaz](Triaz = 1,4-二苯基-3-甲基-1,2,3-三唑通过三甲基甲硅烷基保护的3-甲基-4-苯基-1,2,3-碘化三唑鎓碘化功能的氧化石墨烯材料TRGO-的金属化制备通过三唑基亚烷基接头共价固定的-5-亚基铑(III)络合物Triaz和[Cp * RhCl 2 ] 2(使用钠叔)-丁醇为碱。通过XPS和扩展X射线吸收精细结构(EXAFS)光谱测定了负载的铑(III)配合物的配位范围,表明碳壁上的O-官能团取代了碘配体。与均相催化剂形成鲜明对比的是,尽管均相催化剂TRGO-Triaz-Rh(III)在60°C时表现出出色的催化性能,但它在室温下没有活性。此外,杂化催化剂TRGO-Triaz-Rh(III)具有出色的可回收性,在丙酮对-1--1-炔与HSiMePh 2的氢化硅烷化反应中,对β-(Z)-乙烯基硅烷产品。已经通过DFT计算探索了分子催化剂[Cp * RhI(C,C')-Triaz]的反应机理,指出了一种金属-配体双功能机理,该机理涉及可逆的环金属化,与非合作途径竞争。拟议的机制需要Rh-C Ar辅助的氢硅烷活化,以提供反应性的Rh-甲硅烷基中间体,从而在炔烃插入和金属环丙烯驱动的异构化后生成(E)-甲硅烷基亚乙烯基中间体。β-(Z)-乙烯基硅烷产物的释放可以通过涉及σ-CAM和C Ar的可逆环金属化机理发生-H键或外部氢硅烷的Si-H键。后者的能垒比C Ar -H键低1.2 kcal·mol –1,这导致较小的能量跨度差异,使两种途径在催化条件下都具有竞争性。
更新日期:2020-11-21
中文翻译:

环金属化铑(III)-三唑基均相和均相末端炔烃加氢硅烷化催化剂控制β-(Z)选择性
环金属化的Rh(III)-NHC化合物[Cp * RhI(C,C')-Triaz](Triaz = 1,4-二苯基-3-甲基-1,2,3-三唑-5-亚萘基)和[Cp * RhI(C,C')-Im](Im = 1-苯基-3-甲基-咪唑-2-亚烷基)是有效的催化剂,可用于末端炔烃的氢化硅烷化,对热力学不稳定的β-具有完全的区域和立体选择性(Z)-乙烯基硅烷异构体在室温下在氯仿或丙酮中。催化剂[Cp * RhI(C,C')-Triaz]在活性方面表现出优异的催化性能,已被应用于各种线性1-炔烃和苯基乙炔衍生物与各种氢化硅烷(包括HSiMePh 2,HSiMe)的氢化硅烷化反应2相,HSiEt 3,以及体积较大的七甲基氢三硅氧烷(HMTS),以定量收率得到相应的β-(Z)-乙烯基硅烷。石墨烯基杂化材料TRGO-Triaz-Rh(III),具有环金属化的[Cp * RhI(C,C')-Triaz](Triaz = 1,4-二苯基-3-甲基-1,2,3-三唑通过三甲基甲硅烷基保护的3-甲基-4-苯基-1,2,3-碘化三唑鎓碘化功能的氧化石墨烯材料TRGO-的金属化制备通过三唑基亚烷基接头共价固定的-5-亚基铑(III)络合物Triaz和[Cp * RhCl 2 ] 2(使用钠叔)-丁醇为碱。通过XPS和扩展X射线吸收精细结构(EXAFS)光谱测定了负载的铑(III)配合物的配位范围,表明碳壁上的O-官能团取代了碘配体。与均相催化剂形成鲜明对比的是,尽管均相催化剂TRGO-Triaz-Rh(III)在60°C时表现出出色的催化性能,但它在室温下没有活性。此外,杂化催化剂TRGO-Triaz-Rh(III)具有出色的可回收性,在丙酮对-1--1-炔与HSiMePh 2的氢化硅烷化反应中,对β-(Z)-乙烯基硅烷产品。已经通过DFT计算探索了分子催化剂[Cp * RhI(C,C')-Triaz]的反应机理,指出了一种金属-配体双功能机理,该机理涉及可逆的环金属化,与非合作途径竞争。拟议的机制需要Rh-C Ar辅助的氢硅烷活化,以提供反应性的Rh-甲硅烷基中间体,从而在炔烃插入和金属环丙烯驱动的异构化后生成(E)-甲硅烷基亚乙烯基中间体。β-(Z)-乙烯基硅烷产物的释放可以通过涉及σ-CAM和C Ar的可逆环金属化机理发生-H键或外部氢硅烷的Si-H键。后者的能垒比C Ar -H键低1.2 kcal·mol –1,这导致较小的能量跨度差异,使两种途径在催化条件下都具有竞争性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号