Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Candida antarctica lipase‐B‐catalyzed kinetic resolution of 1,3‐dialkyl‐3‐hydroxymethyl oxindoles
Chirality ( IF 2.8 ) Pub Date : 2020-11-03 , DOI: 10.1002/chir.23284 Naveen Kumar 1 , Akshay Kumar 2 , Subash Chandra Sahoo 3 , Swapandeep Singh Chimni 1
Chirality ( IF 2.8 ) Pub Date : 2020-11-03 , DOI: 10.1002/chir.23284 Naveen Kumar 1 , Akshay Kumar 2 , Subash Chandra Sahoo 3 , Swapandeep Singh Chimni 1
Affiliation
|
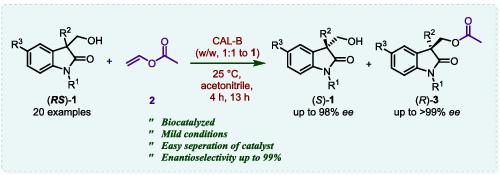
Candida antarctica (CAL‐B) lipase‐catalyzed resolution of 1,3‐dialkyl‐3‐hydroxymethyl oxindoles has been performed to obtain (R)‐1,3‐dialkyl‐3‐acetoxymethyl oxindoles with up to 99% ee and (S)‐1,3‐dialkyl‐3‐hydroxymethyl oxindoles with up to 78% ee using vinyl acetate as acylating agent and acetonitrile as solvent transforming (S)‐3‐allyl‐3‐hydroxymethyl oxindole to (3S)‐1′‐benzyl‐5‐(iodomethyl)‐4,5‐dihydro‐2H‐spiro[furan‐3,3′‐indolin]‐2′‐one. The optically active 3‐substituted‐3‐hydroxymethyl oxindoles and spiro‐oxindoles are among the key synthons in the synthesis of potentially biologically active molecules.
中文翻译:

南极念珠菌脂肪酶-B催化动力学拆分1,3-二烷基-3-羟甲基羟吲哚
南极念珠菌(CAL-B) 脂肪酶催化拆分 1,3-二烷基-3-羟甲基羟甲基羟吲哚,获得 ( R )-1,3-二烷基-3-乙酰氧基甲基羟吲哚,其ee和 ( S )-1,3-二烷基-3-羟甲基羟甲基羟吲哚,具有高达 78% ee使用乙酸乙烯酯作为酰化剂和乙腈作为溶剂将 ( S )-3-烯丙基-3-羟甲基羟甲基羟吲哚转化为 ( 3S )-1'- benzyl-5-(iodomethyl)-4,5-dihydro-2 H - spiro[furan-3,3'-indolin]-2'-one。光学活性的 3-取代-3-羟甲基羟吲哚和螺-羟吲哚是合成潜在生物活性分子的关键合成子。
更新日期:2020-11-21
中文翻译:

南极念珠菌脂肪酶-B催化动力学拆分1,3-二烷基-3-羟甲基羟吲哚
南极念珠菌(CAL-B) 脂肪酶催化拆分 1,3-二烷基-3-羟甲基羟甲基羟吲哚,获得 ( R )-1,3-二烷基-3-乙酰氧基甲基羟吲哚,其ee和 ( S )-1,3-二烷基-3-羟甲基羟甲基羟吲哚,具有高达 78% ee使用乙酸乙烯酯作为酰化剂和乙腈作为溶剂将 ( S )-3-烯丙基-3-羟甲基羟甲基羟吲哚转化为 ( 3S )-1'- benzyl-5-(iodomethyl)-4,5-dihydro-2 H - spiro[furan-3,3'-indolin]-2'-one。光学活性的 3-取代-3-羟甲基羟吲哚和螺-羟吲哚是合成潜在生物活性分子的关键合成子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号