当前位置:
X-MOL 学术
›
Steel Res. Int.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mineral Transform and Specific Heat Capacity Characterization of Blast Furnace Slag with High Al2O3 in Heating Process
Steel Research International ( IF 1.9 ) Pub Date : 2020-11-03 , DOI: 10.1002/srin.202000448 Haiyan Zheng 1 , Lisheng Liang 2 , Jinlei Du 1 , Shifa Zhou 1 , Xin Jiang 1 , Qiangjian Gao 1 , Fengman Shen 1
Steel Research International ( IF 1.9 ) Pub Date : 2020-11-03 , DOI: 10.1002/srin.202000448 Haiyan Zheng 1 , Lisheng Liang 2 , Jinlei Du 1 , Shifa Zhou 1 , Xin Jiang 1 , Qiangjian Gao 1 , Fengman Shen 1
Affiliation
|
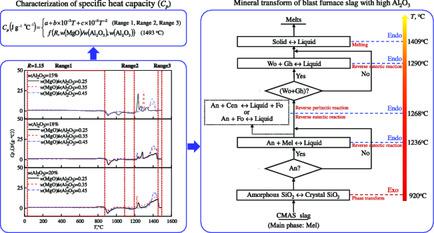
The specific heat capacity (Cp) of blast furnace (BF) slag critically impacts BF smelting and reports on the Cp of BF slag with high Al2O3 are rare. Mineral transform and Cp characterization of synthesized BF slag (CaO–SiO2–MgO–Al2O3) with high Al2O3 in the heating process are herein discussed. Differential scanning calorimetry curves show a major exothermic peak at a low temperature and multiple endothermic peaks at elevated temperatures. The exothermic peak at approximately 920 °C appears when amorphous SiO2 (or SiO2 in silicate) becomes crystal SiO2 (or SiO2 in silicate). The endothermic peak between 1224–1254 °C is attributed to the reverse eutectic reaction, CaO·Al2O3·2SiO2 + 2CaO·0.5MgO·0.5Al2O3·1.5SiO2 → Liquid, along a eutectic line. CaO·Al2O3·2SiO2 + MgO·SiO2 → Liquid + 2MgO·SiO2 (reverse peritectic reaction) or CaO·Al2O3·2SiO2 + 2MgO·SiO2 → Liquid (reverse eutectic reaction) at approximately 1268 °C will occur with an endothermic reaction in some cases. The sharp endothermic peak between 1282 and 1303 °C is the reverse eutectic reaction, CaO·SiO2 + 2CaO·Al2O3·SiO2 → Liquid. The blunt endothermic peak between 1388 and 1447 °C is the residual slag melting, Solid → Liquid. The relationships of Cp with temperatures and compositions (R, ratio of w(MgO)/w(Al2O3), and w(Al2O3)) are established with a good fit.
中文翻译:

高Al2O3高炉炉渣加热过程的矿物转化及比热容表征。
高炉渣的比热容(C p)对高炉冶炼产生重大影响,关于Al 2 O 3含量高的高炉渣C p的报道很少。本文讨论了在加热过程中具有高Al 2 O 3的高炉渣(CaO–SiO 2 –MgO–Al 2 O 3)的矿物转化和C p表征。差示扫描量热曲线显示了在低温下的主要放热峰和在高温下的多个吸热峰。当无定形SiO 2时,在大约920°C出现放热峰(或硅酸盐中的SiO 2)变成晶体SiO 2(或硅酸盐中的SiO 2)。 沿着共晶线,在1224–1254°C之间的吸热峰归因于反共晶反应,即CaO·Al 2 O 3 ·2SiO 2 + 2CaO·0.5MgO·0.5Al 2 O 3 ·1.5SiO 2 →液体。CaO·Al 2 O 3 ·2SiO 2 + MgO·SiO 2 →液体+ 2MgO·SiO 2(逆包晶反应)或CaO·Al 2 O 3 ·2SiO 2 + 2MgO·SiO 2 →在某些情况下,大约1268°C时会发生液体(逆共晶反应),并发生吸热反应。在1282和1303°C之间的急剧吸热峰是反向共晶反应,CaO·SiO 2 + 2CaO·Al 2 O 3 ·SiO 2 →液体。1388至1447°C之间的钝吸热峰是残余的熔渣融化,即固体→液体。的关系Ç p与温度和组合物([R ,的比值瓦特(MgO)的/瓦特(铝2 ö 3),和瓦特(铝2 ö 3))成立具有良好的配合。
更新日期:2020-11-03
中文翻译:

高Al2O3高炉炉渣加热过程的矿物转化及比热容表征。
高炉渣的比热容(C p)对高炉冶炼产生重大影响,关于Al 2 O 3含量高的高炉渣C p的报道很少。本文讨论了在加热过程中具有高Al 2 O 3的高炉渣(CaO–SiO 2 –MgO–Al 2 O 3)的矿物转化和C p表征。差示扫描量热曲线显示了在低温下的主要放热峰和在高温下的多个吸热峰。当无定形SiO 2时,在大约920°C出现放热峰(或硅酸盐中的SiO 2)变成晶体SiO 2(或硅酸盐中的SiO 2)。 沿着共晶线,在1224–1254°C之间的吸热峰归因于反共晶反应,即CaO·Al 2 O 3 ·2SiO 2 + 2CaO·0.5MgO·0.5Al 2 O 3 ·1.5SiO 2 →液体。CaO·Al 2 O 3 ·2SiO 2 + MgO·SiO 2 →液体+ 2MgO·SiO 2(逆包晶反应)或CaO·Al 2 O 3 ·2SiO 2 + 2MgO·SiO 2 →在某些情况下,大约1268°C时会发生液体(逆共晶反应),并发生吸热反应。在1282和1303°C之间的急剧吸热峰是反向共晶反应,CaO·SiO 2 + 2CaO·Al 2 O 3 ·SiO 2 →液体。1388至1447°C之间的钝吸热峰是残余的熔渣融化,即固体→液体。的关系Ç p与温度和组合物([R ,的比值瓦特(MgO)的/瓦特(铝2 ö 3),和瓦特(铝2 ö 3))成立具有良好的配合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号