当前位置:
X-MOL 学术
›
Stem Cells Transl. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lithium modulates miR‐1906 levels of mesenchymal stem cell‐derived extracellular vesicles contributing to poststroke neuroprotection by toll‐like receptor 4 regulation
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2020-11-04 , DOI: 10.1002/sctm.20-0086 Matteo Haupt 1 , Xuan Zheng 1 , Yaoyun Kuang 1 , Simone Lieschke 1 , Lisa Janssen 1 , Bert Bosche 2, 3, 4 , Fengyan Jin 5 , Katharina Hein 1 , Ertugrul Kilic 6 , Vivek Venkataramani 7 , Dirk M Hermann 3 , Mathias Bähr 1 , Thorsten R Doeppner 1, 3, 6
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2020-11-04 , DOI: 10.1002/sctm.20-0086 Matteo Haupt 1 , Xuan Zheng 1 , Yaoyun Kuang 1 , Simone Lieschke 1 , Lisa Janssen 1 , Bert Bosche 2, 3, 4 , Fengyan Jin 5 , Katharina Hein 1 , Ertugrul Kilic 6 , Vivek Venkataramani 7 , Dirk M Hermann 3 , Mathias Bähr 1 , Thorsten R Doeppner 1, 3, 6
Affiliation
|
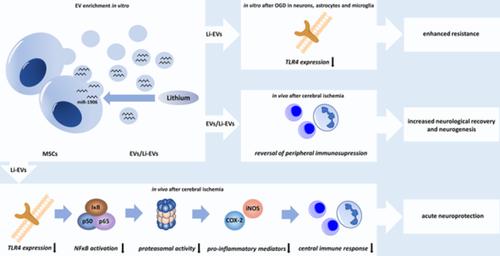
Lithium is neuroprotective in preclinical stroke models. In addition to that, poststroke neuroregeneration is stimulated upon transplantation of mesenchymal stem cells (MSCs). Preconditioning of MSCs with lithium further enhances the neuroregenerative potential of MSCs, which act by secreting extracellular vesicles (EVs). The present work analyzed whether MSC preconditioning with lithium modifies EV secretion patterns, enhancing the therapeutic potential of such derived EVs (Li‐EVs) in comparison with EVs enriched from native MSCs. Indeed, Li‐EVs significantly enhanced the resistance of cultured astrocytes, microglia, and neurons against hypoxic injury when compared with controls and to native EV‐treated cells. Using a stroke mouse model, intravenous delivery of Li‐EVs increased neurological recovery and neuroregeneration for as long as 3 months in comparison with controls and EV‐treated mice, albeit the latter also showed significantly better behavioral test performance compared with controls. Preconditioning of MSCs with lithium also changed the secretion patterns for such EVs, modifying the contents of various miRNAs within these vesicles. As such, Li‐EVs displayed significantly increased levels of miR‐1906, which has been shown to be a new regulator of toll‐like receptor 4 (TLR4) signaling. Li‐EVs reduced posthypoxic and postischemic TLR4 abundance, resulting in an inhibition of the nuclear factor kappa‐light‐chain‐enhancer of activated B cells (NF‐κB) signaling pathway, decreased proteasomal activity, and declined both inducible NO synthase and cyclooxygenase‐2 expression, all of which culminated in reduced levels of poststroke cerebral inflammation. Conclusively, the present study demonstrates, for the first time, an enhanced therapeutic potential of Li‐EVs compared with native EVs, interfering with a novel signaling pathway that yields both acute neuroprotection and enhanced neurological recovery.
中文翻译:

锂通过 toll 样受体 4 调节调节间充质干细胞衍生的细胞外囊泡的 miR-1906 水平,促进卒中后神经保护
锂在临床前卒中模型中具有神经保护作用。除此之外,间充质干细胞 (MSCs) 移植可刺激中风后神经再生。用锂对 MSCs 进行预处理进一步增强了 MSCs 的神经再生潜力,MSCs 通过分泌细胞外囊泡 (EVs) 发挥作用。目前的工作分析了与富含天然 MSC 的 EV 相比,用锂进行 MSC 预处理是否会改变 EV 分泌模式,从而增强此类衍生 EV (Li-EV) 的治疗潜力。事实上,与对照和天然 EV 处理的细胞相比,Li-EV 显着增强了培养的星形胶质细胞、小胶质细胞和神经元对缺氧损伤的抵抗力。使用中风鼠标模型,与对照组和 EV 治疗的小鼠相比,Li-EVs 的静脉内给药增加了长达 3 个月的神经恢复和神经再生,尽管与对照组相比,后者也表现出明显更好的行为测试性能。用锂对 MSC 进行预处理也改变了此类 EV 的分泌模式,改变了这些囊泡内各种 miRNA 的含量。因此,Li-EV 的 miR-1906 水平显着增加,已被证明是 toll 样受体 4 (TLR4) 信号传导的新调节剂。Li-EVs 降低了缺氧后和缺血后 TLR4 的丰度,导致活化 B 细胞 (NF-κB) 信号通路的核因子 kappa-轻链增强子 (NF-κB) 信号通路受到抑制,蛋白酶体活性降低,诱导型 NO 合酶和环氧合酶均下降。 2 表达式,所有这些最终导致中风后脑炎症水平降低。总之,本研究首次证明,与天然 EV 相比,Li-EV 的治疗潜力增强,干扰了一种新的信号通路,可产生急性神经保护和增强的神经恢复。
更新日期:2020-11-04
中文翻译:

锂通过 toll 样受体 4 调节调节间充质干细胞衍生的细胞外囊泡的 miR-1906 水平,促进卒中后神经保护
锂在临床前卒中模型中具有神经保护作用。除此之外,间充质干细胞 (MSCs) 移植可刺激中风后神经再生。用锂对 MSCs 进行预处理进一步增强了 MSCs 的神经再生潜力,MSCs 通过分泌细胞外囊泡 (EVs) 发挥作用。目前的工作分析了与富含天然 MSC 的 EV 相比,用锂进行 MSC 预处理是否会改变 EV 分泌模式,从而增强此类衍生 EV (Li-EV) 的治疗潜力。事实上,与对照和天然 EV 处理的细胞相比,Li-EV 显着增强了培养的星形胶质细胞、小胶质细胞和神经元对缺氧损伤的抵抗力。使用中风鼠标模型,与对照组和 EV 治疗的小鼠相比,Li-EVs 的静脉内给药增加了长达 3 个月的神经恢复和神经再生,尽管与对照组相比,后者也表现出明显更好的行为测试性能。用锂对 MSC 进行预处理也改变了此类 EV 的分泌模式,改变了这些囊泡内各种 miRNA 的含量。因此,Li-EV 的 miR-1906 水平显着增加,已被证明是 toll 样受体 4 (TLR4) 信号传导的新调节剂。Li-EVs 降低了缺氧后和缺血后 TLR4 的丰度,导致活化 B 细胞 (NF-κB) 信号通路的核因子 kappa-轻链增强子 (NF-κB) 信号通路受到抑制,蛋白酶体活性降低,诱导型 NO 合酶和环氧合酶均下降。 2 表达式,所有这些最终导致中风后脑炎症水平降低。总之,本研究首次证明,与天然 EV 相比,Li-EV 的治疗潜力增强,干扰了一种新的信号通路,可产生急性神经保护和增强的神经恢复。











































 京公网安备 11010802027423号
京公网安备 11010802027423号