当前位置:
X-MOL 学术
›
Propellants Explos. Pyrotech.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Optimization of Synthesis Parameters and Characterization of Green Primary Explosive Copper(I) 5‐Nitrotetrazolate (DBX‐1)
Propellants, Explosives, Pyrotechnics ( IF 1.7 ) Pub Date : 2020-11-04 , DOI: 10.1002/prep.202000173 Wen‐Hsiang Li, Kai‐Chun Tseng, Tsung‐Mao Yang, Jin‐Shuh Li, Kai‐Tai Lu
Propellants, Explosives, Pyrotechnics ( IF 1.7 ) Pub Date : 2020-11-04 , DOI: 10.1002/prep.202000173 Wen‐Hsiang Li, Kai‐Chun Tseng, Tsung‐Mao Yang, Jin‐Shuh Li, Kai‐Tai Lu
|
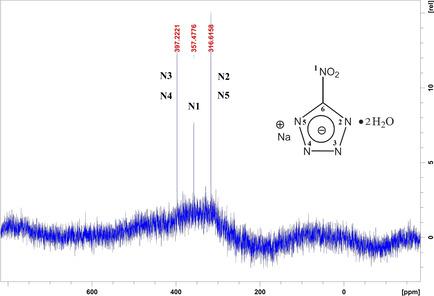
Since the early 20th century, lead azide (LA) has been commonly used as a primary explosive. However, lead pollution in the air and soil has attracted more and more attention, particularly in military training grounds and shooting ranges. Copper(I) 5‐nitrotetrazolate (DBX‐1) is considered as one of the most promising alternatives to LA. DBX‐1 is typically prepared from sodium 5‐nitrotetrazolate dihydrate [NaNT(H2O)2] and copper(I) chloride (CuCl). But little is known about its optimal synthesis parameters. In addition, NaNT(H2O)2 is not commercially available. In this study, NaNT(H2O)2 was prepared by ourselves. Taguchi's experimental design method was used to determine the optimal experimental conditions for obtaining the maximum yield of DBX‐1. The synthesized NaNT(H2O)2 and DBX‐1 were identified by means of SEM, NMR, FTIR, EA, UV‐Vis and STA TG‐DSC, and the sensitivity of DBX‐1 was determined using BAM fallhammer, BAM friction tester and electrostatic spark sensitivity tester. The experimental results indicated that the optimal synthesis parameters of DBX‐1 were as follows: the reaction temperature was 100 °C, the reaction time was 30 min, the concentration of NaNT(H2O)2 was 0.075 wt.% and the molar ratio of NaNT(H2O)2 to CuCl was 1.15, and then the maximum yield after purification could reach 72.2 %. The decomposition activation energies of DBX‐1 calculated by Kissinger and Ozawa methods were 178.6 and 179.0 kJ/mol, respectively. In addition, the impact sensitivity, friction sensitivity and electrostatic spark sensitivity of DBX‐1 were 51 mJ, 0.4 N and 7.3 mJ, respectively, which were almost the same as those for LA.
中文翻译:

绿色初生炸药5-硝基四氮杂铜(DBX-1)的合成参数优化和表征
自20世纪初期以来,叠氮化铅(LA)一直被用作主要炸药。但是,空气和土壤中的铅污染已引起越来越多的关注,特别是在军事训练场和射击场中。5-硝基四唑酸铜(I)(DBX-1)被认为是洛杉矶最有希望的替代品之一。DBX-1通常由5-硝基四唑酸钠二水合物[NaNT(H 2 O)2 ]和氯化铜(I)(CuCl)制备。但是对其最佳合成参数知之甚少。另外,NaNT(H 2 O)2不可商购。在这项研究中,NaNT(H 2 O)2由我们自己准备。Taguchi的实验设计方法用于确定获得DBX-1最高产量的最佳实验条件。通过SEM,NMR,FTIR,EA,UV-Vis和STA TG-DSC对合成的NaNT(H 2 O)2和DBX-1进行鉴定,并使用BAM落锤,BAM摩擦测定DBX-1的灵敏度测试仪和静电火花灵敏度测试仪。实验结果表明,DBX-1的最佳合成参数如下:反应温度为100°C,反应时间为30 min,NaNT(H 2 O)2的浓度为0.075 wt。%,摩尔比NaNT(H 2 O)2的比例CuCl的最大收率为1.15,纯化后的最大收率可达72.2%。用Kissinger和Ozawa方法计算出的DBX-1的分解活化能分别为178.6和179.0 kJ / mol。此外,DBX-1的冲击灵敏度,摩擦灵敏度和静电火花灵敏度分别为51 mJ,0.4 N和7.3 mJ,与LA几乎相同。
更新日期:2020-12-07
中文翻译:

绿色初生炸药5-硝基四氮杂铜(DBX-1)的合成参数优化和表征
自20世纪初期以来,叠氮化铅(LA)一直被用作主要炸药。但是,空气和土壤中的铅污染已引起越来越多的关注,特别是在军事训练场和射击场中。5-硝基四唑酸铜(I)(DBX-1)被认为是洛杉矶最有希望的替代品之一。DBX-1通常由5-硝基四唑酸钠二水合物[NaNT(H 2 O)2 ]和氯化铜(I)(CuCl)制备。但是对其最佳合成参数知之甚少。另外,NaNT(H 2 O)2不可商购。在这项研究中,NaNT(H 2 O)2由我们自己准备。Taguchi的实验设计方法用于确定获得DBX-1最高产量的最佳实验条件。通过SEM,NMR,FTIR,EA,UV-Vis和STA TG-DSC对合成的NaNT(H 2 O)2和DBX-1进行鉴定,并使用BAM落锤,BAM摩擦测定DBX-1的灵敏度测试仪和静电火花灵敏度测试仪。实验结果表明,DBX-1的最佳合成参数如下:反应温度为100°C,反应时间为30 min,NaNT(H 2 O)2的浓度为0.075 wt。%,摩尔比NaNT(H 2 O)2的比例CuCl的最大收率为1.15,纯化后的最大收率可达72.2%。用Kissinger和Ozawa方法计算出的DBX-1的分解活化能分别为178.6和179.0 kJ / mol。此外,DBX-1的冲击灵敏度,摩擦灵敏度和静电火花灵敏度分别为51 mJ,0.4 N和7.3 mJ,与LA几乎相同。











































 京公网安备 11010802027423号
京公网安备 11010802027423号