当前位置:
X-MOL 学术
›
ChemPhysChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
DFT and Empirical Considerations on Electrocatalytic Water/Carbon Dioxide Reduction by CoTMPyP in Neutral Aqueous Solutions**
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-11-03 , DOI: 10.1002/cphc.202000715 Yair Bochlin 1 , Yeshayahu Ben‐Eliyahu 2 , Yanir Kadosh 1 , Sebastian Kozuch 3 , Israel Zilbermann 2, 3 , Eli Korin 1 , Armand Bettelheim 1
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-11-03 , DOI: 10.1002/cphc.202000715 Yair Bochlin 1 , Yeshayahu Ben‐Eliyahu 2 , Yanir Kadosh 1 , Sebastian Kozuch 3 , Israel Zilbermann 2, 3 , Eli Korin 1 , Armand Bettelheim 1
Affiliation
|
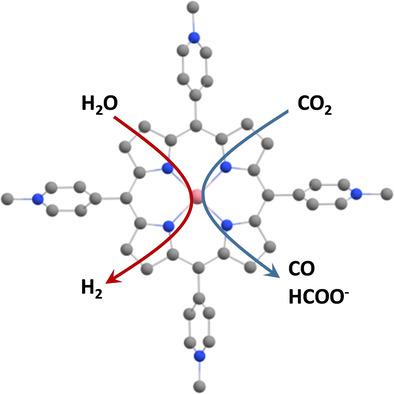
A combined experimental and density functional theory (DFT) investigation was employed in order to examine the mechanism of electrochemical CO2 reduction and H2 formation from water reduction in neutral aqueous solutions. A water soluble cobalt porphyrin, cobalt [5,10,15,20‐(tetra‐N‐methyl‐4‐pyridyl)porphyrin], (CoTMPyP), was used as catalyst. The possible attachment of different axial ligands as well as their effect on the electrocatalytic cycles were examined. A cobalt porphyrin hydride is a key intermediate which is generated after the initial reduction of the catalyst. The hydride is involved in the formation of H2 and formate and acts as an indirect proton source for the formation of CO in these H+‐starving conditions. The experimental results are in agreement with the computations and give new insights into electrocatalytic mechanisms involving water soluble metalloporphyrins. We conclude that in addition to the porphyrin's structure and metal ion center, the electrolyte surroundings play a key role in dictating the products of CO2/H2O reduction.
中文翻译:

在中性水溶液中CoTMPyP还原电催化水/二氧化碳的DFT和经验性考虑**
为了研究中性水溶液中水还原时电化学还原CO 2和生成H 2的机理,采用了结合实验和密度泛函理论(DFT)的研究方法。水溶性钴卟啉钴[5,10,15,20-(四-N-甲基-4-吡啶基)卟啉](CoTMPyP)被用作催化剂。研究了不同轴向配体的可能连接以及它们对电催化循环的影响。氢化卟啉钴是关键的中间体,它是在催化剂初次还原后产生的。氢化物参与H 2和甲酸酯的形成,并作为这些H +中CO形成的间接质子源饥饿的条件。实验结果与计算结果一致,并对涉及水溶性金属卟啉的电催化机理提供了新的见解。我们得出的结论是,除了卟啉的结构和金属离子中心外,电解质的周围环境在决定CO 2 / H 2 O还原产物中起着关键作用。
更新日期:2020-12-17
中文翻译:

在中性水溶液中CoTMPyP还原电催化水/二氧化碳的DFT和经验性考虑**
为了研究中性水溶液中水还原时电化学还原CO 2和生成H 2的机理,采用了结合实验和密度泛函理论(DFT)的研究方法。水溶性钴卟啉钴[5,10,15,20-(四-N-甲基-4-吡啶基)卟啉](CoTMPyP)被用作催化剂。研究了不同轴向配体的可能连接以及它们对电催化循环的影响。氢化卟啉钴是关键的中间体,它是在催化剂初次还原后产生的。氢化物参与H 2和甲酸酯的形成,并作为这些H +中CO形成的间接质子源饥饿的条件。实验结果与计算结果一致,并对涉及水溶性金属卟啉的电催化机理提供了新的见解。我们得出的结论是,除了卟啉的结构和金属离子中心外,电解质的周围环境在决定CO 2 / H 2 O还原产物中起着关键作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号