Molecular Cell ( IF 14.5 ) Pub Date : 2020-11-04 , DOI: 10.1016/j.molcel.2020.10.013 Yoshihiro Adachi 1 , Takashi Kato 1 , Tatsuya Yamada 1 , Daisuke Murata 1 , Kenta Arai 1 , Robert V Stahelin 2 , David C Chan 3 , Miho Iijima 1 , Hiromi Sesaki 1
|
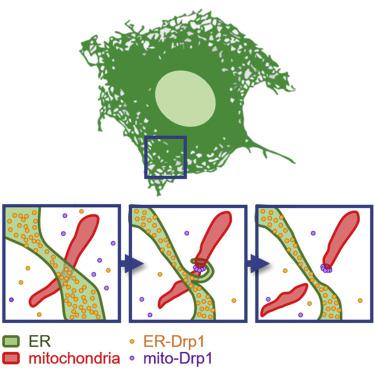
Mitochondria are highly dynamic organelles that continuously grow, divide, and fuse. The division of mitochondria is crucial for human health. During mitochondrial division, the mechano-guanosine triphosphatase (GTPase) dynamin-related protein (Drp1) severs mitochondria at endoplasmic reticulum (ER)-mitochondria contact sites, where peripheral ER tubules interact with mitochondria. Here, we report that Drp1 directly shapes peripheral ER tubules in human and mouse cells. This ER-shaping activity is independent of GTP hydrolysis and located in a highly conserved peptide of 18 amino acids (termed D-octadecapeptide), which is predicted to form an amphipathic α helix. Synthetic D-octadecapeptide tubulates liposomes in vitro and the ER in cells. ER tubules formed by Drp1 promote mitochondrial division by facilitating ER-mitochondria interactions. Thus, Drp1 functions as a two-in-one protein during mitochondrial division, with ER tubulation and mechano-GTPase activities.
中文翻译:

Drp1 以独立于 GTPase 的方式管状内质网
线粒体是高度动态的细胞器,不断生长、分裂和融合。线粒体的分裂对人类健康至关重要。在线粒体分裂过程中,机械-鸟苷三磷酸酶 (GTPase) 动力蛋白相关蛋白 (Drp1) 在内质网 (ER)-线粒体接触位点切断线粒体,外周 ER 小管与线粒体相互作用。在这里,我们报告 Drp1 直接塑造人和小鼠细胞中的外周 ER 小管。这种 ER 整形活性不依赖于 GTP 水解,并且位于高度保守的 18 个氨基酸的肽(称为 D-十八肽)中,预计会形成两亲性 α 螺旋。体外合成的 D-十八肽管状脂质体和细胞中的ER。Drp1 形成的 ER 小管通过促进 ER-线粒体相互作用来促进线粒体分裂。因此,Drp1 在线粒体分裂过程中作为二合一蛋白发挥作用,具有 ER 管和机械 GTPase 活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号