Matrix Biology ( IF 4.5 ) Pub Date : 2020-11-04 , DOI: 10.1016/j.matbio.2020.10.006 Zaki Al-Yafeai 1 , Brenna H Pearson 1 , Jonette M Peretik 2 , Elizabeth D Cockerham 2 , Kaylea A Reeves 2 , Umesh Bhattarai 1 , Dongdong Wang 2 , Brian G Petrich 3 , A Wayne Orr 4
|
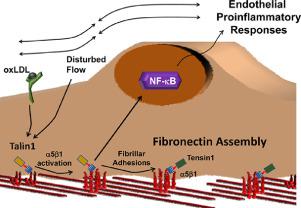
While vital to platelet and leukocyte adhesion, the role of integrin affinity modulation in adherent cells remains controversial. In endothelial cells, atheroprone hemodynamics and oxidized lipoproteins drive an increase in the high affinity conformation of α5β1 integrins in endothelial cells in vitro, and α5β1 integrin inhibitors reduce proinflammatory endothelial activation to these stimuli in vitro and in vivo. However, the importance of α5β1 integrin affinity modulation to endothelial phenotype remains unknown. We now show that endothelial cells (talin1 L325R) unable to induce high affinity integrins initially adhere and spread but show significant defects in nascent adhesion formation. In contrast, overall focal adhesion number, area, and composition in stably adherent cells are similar between talin1 wildtype and talin1 L325R endothelial cells. However, talin1 L325R endothelial cells fail to induce high affinity α5β1 integrins, fibronectin deposition, and proinflammatory responses to atheroprone hemodynamics and oxidized lipoproteins. Inducing the high affinity conformation of α5β1 integrins in talin1 L325R endothelial cells suggest that NF-κB activation and maximal fibronectin deposition require both integrin activation and other integrin-independent signaling. In endothelial-specific talin1 L325R mice, atheroprone hemodynamics fail to promote inflammation and macrophage recruitment, demonstrating a vital role for integrin activation in regulating endothelial phenotype.
中文翻译:

整合素亲和力调节在体外和体内关键调节致动脉粥样硬化内皮活化
虽然对血小板和白细胞粘附至关重要,但整合素亲和力调节在粘附细胞中的作用仍然存在争议。在内皮细胞中,动脉粥样硬化倾向的血流动力学和氧化的脂蛋白在体外驱动内皮细胞中 α5β1 整合素的高亲和力构象增加,而 α5β1 整合素抑制剂在体外和体内减少对这些刺激的促炎内皮激活. 然而,α5β1 整合素亲和力调节对内皮表型的重要性仍然未知。我们现在表明,内皮细胞 (talin1 L325R) 最初无法诱导高亲和力整合素粘附和扩散,但在新生粘附形成中显示出显着缺陷。相比之下,在 talin1 野生型和 talin1 L325R 内皮细胞之间,稳定粘附细胞中的总粘着斑数量、面积和组成相似。然而,talin1 L325R 内皮细胞不能诱导高亲和力 α5β1 整合素、纤连蛋白沉积和对动脉粥样硬化倾向的血流动力学和氧化脂蛋白的促炎反应。在 talin1 L325R 内皮细胞中诱导 α5β1 整合素的高亲和力构象表明 NF-κB 激活和最大纤连蛋白沉积需要整合素激活和其他整合素非依赖性信号传导。在内皮特异性 talin1 L325R 小鼠中,atheroprone 血流动力学不能促进炎症和巨噬细胞募集,这表明整合素激活在调节内皮表型中起着至关重要的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号