European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2020-11-04 , DOI: 10.1016/j.ejmech.2020.112984 Lin An , Chan Wang , You-Guang Zheng , Jia-dong Liu , Tong-hui Huang
|
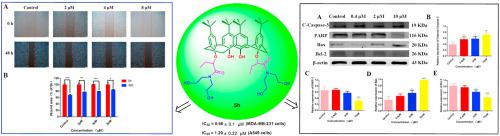
Calixarenes, with potential functionalization on the upper and lower rim, have been explored in recent years for the design and construction of anticancer agents in the field of drugs and pharmaceuticals. Herein, optimization of bis [N-(2-hydroxyethyl) aminocarbonylmethoxyl substituted calix[4] arene (CLX-4) using structure-based drug design and traditional medicinal chemistry led to the discovery of series of calix[4]arene carbonyl amide derivatives 5a-5t. Evaluation of the cytotoxicity of 5a-5t employing MTT assay in MCF-7, MDA-MB-231 (human breast cancer cells), HT29 (human colon carcinoma cells), HepG2 (human hepatocellular carcinoma cells), A549 (human lung adenocarcinoma cells) and HUVEC (Human Umbilical Vein Endothelial) cells demonstrated that the most promising compound 5h displayed the most superior inhibitory effect against A549 and MDA-MB-231 cells, which were 3.2 times and 6.8 times of CLX-4, respectively. In addition, the cell inhibition rate (at 10 μM) against normal HUVEC cells in vitro was only 9.6%, indicating the safty of compound 5h. Moreover, compound 5h could inhibit the migration of MDA-MB-231 cell in wound healing assay. Further mechanism studies significantly indicated that compound 5h could block MDA-MB-231 cell cycle arrest in G0/G1 phase by down regulating cyclin D1 and CDK4, and induce apoptosis by up-regulation of Bax, down-regulation of Caspase-3, PARP and Bcl-2 proteins, resulting in the reduction of DNA synthesis and cell division arrest. This work provides worthy of further exploration for the promising calixarene-based anticancer drugs.
中文翻译:

杯[4]芳烃基具有抗肿瘤活性的羰基酰胺衍生物的设计,合成与评价
近年来,对于在药物和药物领域中抗癌剂的设计和构建,人们已经探索了具有上下边缘潜在功能的杯芳烃。在此,使用基于结构的药物设计和传统药物化学优化双[N-(2-羟乙基)氨基羰基甲氧基取代的杯[4]芳烃(CLX-4),导致发现了杯[4]芳烃羰基酰胺衍生物系列5a-5t。5a-5t细胞毒性的评估在MCF-7,MDA-MB-231(人类乳腺癌细胞),HT29(人类结肠癌细胞),HepG2(人类肝癌细胞),A549(人类肺腺癌细胞)和HUVEC(人类脐静脉内皮细胞)中采用MTT分析)细胞表明,最有希望的化合物5h对A549和MDA-MB-231细胞的抑制作用最强,分别是CLX-4的3.2倍和6.8倍。另外,体外对正常HUVEC细胞的细胞抑制率(在10μM下)仅为9.6%,表明化合物5h的安全性。此外,化合物5h在伤口愈合试验中可以抑制MDA-MB-231细胞的迁移。进一步的机理研究表明,化合物5h可能通过下调细胞周期蛋白D1和CDK4来阻止MDA-MB-231细胞周期阻滞在G0 / G1期,并通过上调Bax,下调Caspase-3,PARP和Bcl-2蛋白诱导凋亡。 DNA合成减少和细胞分裂停滞。这项工作值得对有希望的基于杯芳烃的抗癌药物进行进一步的探索。



























 京公网安备 11010802027423号
京公网安备 11010802027423号