European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-11-04 , DOI: 10.1016/j.ejmech.2020.112985 David S.P. Cardoso , Annamária Kincses , Márta Nové , Gabriella Spengler , Silva Mulhovo , João Aires-de-Sousa , Daniel J.V.A. dos Santos , Maria-José U. Ferreira
|
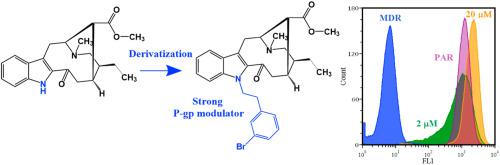
Aiming at generating a series of monoterpene indole alkaloids with enhanced multidrug resistance (MDR) reversing activity in cancer, two major epimeric alkaloids isolated from Tabernaemontana elegans, tabernaemontanine (1) and dregamine (2), were derivatized by alkylation of the indole nitrogen. Twenty-six new derivatives (3-28) were prepared by reaction with different aliphatic and aromatic halides, whose structures were elucidated mainly by NMR, including 2D NMR experiments. Their MDR reversal ability was evaluated through a functional assay, using as models resistant human colon adenocarcinoma and human ABCB1-gene transfected L5178Y mouse lymphoma cells, overexpressing P-glycoprotein (P-gp), by flow cytometry. A considerable increase of activity was found for most of the derivatives, being the strongest P-gp inhibitors those sharing N-phenethyl moieties, displaying outstanding inhibitory activity, associated with weak cytotoxicity. Chemosensitivity assays were also performed in a model of combination chemotherapy in the same cell lines, by studying the in vitro interactions between the compounds and the antineoplastic drug doxorubicin. Most of the compounds have shown strong synergistic interactions with doxorubicin, highlighting their potential as MDR reversers. QSAR models were also explored for insights on drug-receptor interaction, and it was found that lipophilicity and bulkiness features were associated with inhibitory activity, although linear correlations were not observed.
中文翻译:

烷基化的单萜吲哚生物碱衍生物作为抗性癌细胞中有效的P-糖蛋白抑制剂
为了产生一系列在癌症中具有增强的多药耐药性(MDR)逆转活性的单萜吲哚生物碱,通过吲哚氮烷基化衍生化了从线虫属植物Tabernaemontana elegans(1)和dregamine(2)中分离出的两种主要的差向异构生物碱。通过与不同的脂肪族和芳香族卤化物反应制备了26种新的衍生物(3-28),其结构主要通过NMR进行了阐明,包括2D NMR实验。通过功能测定法评估了它们的MDR逆转能力,使用抗人结肠腺癌和人ABCB1作为模型流式细胞仪检测基因转染的L5178Y小鼠淋巴瘤细胞,过表达P-糖蛋白(P-gp)。对于大多数衍生物,发现它们是活性最强的P-gp抑制剂,它们共享N-苯乙基部分,显示出显着的抑制活性,与弱的细胞毒性有关,活性显着增加。通过在体外研究在相同的细胞系中联合化疗的模型中也进行了化学敏感性测定化合物与抗肿瘤药阿霉素之间的相互作用。大多数化合物已显示出与阿霉素的强相互作用,突出了其作为MDR逆转剂的潜力。还探索了QSAR模型以了解药物-受体相互作用,发现亲脂性和体积特征与抑制活性相关,尽管未观察到线性相关性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号